-
PDF
- Split View
-
Views
-
Cite
Cite
Tashinga Musonza, Jose Antonio Tschen, Appendiceal malakoplakia masquerading as a cecal mass, Journal of Surgical Case Reports, Volume 2020, Issue 5, May 2020, rjaa140, https://doi.org/10.1093/jscr/rjaa140
Close - Share Icon Share
Abstract
Appendiceal malakoplakia masquerading as a cecal mass is uniquely rare. The presence of an infiltrate of granular eosinophilic macrophages containing Michaelis–Gutmann bodies on histopathology is pathognomonic of malakoplakia. Cutaneous, gastrointestinal and most commonly urogenital malakoplakia is reported in association with an immunocompromised state, infectious, inflammatory and neoplastic processes. Presentation varies from microscopic disease to plaques, nodules, polypoid lesions and small masses. However, a cecal mass postea proven appendiceal malakoplakia deserves special attention. We could not find similar case reports in the English literature. The pathogenesis of malakoplakia is poorly understood, and it is unclear if it is a harbinger of malignancy, a precursor lesion or an inflammatory marker. In the setting of a dominant appendiceal mass, post-treatment endoscopic and tumor marker surveillance is paramount but, however, undefined in contemporary literature.
Introduction
Appendiceal malakoplakia presenting as a cecal mass on high-resolution imaging is a rare clinical entity. Surgical management reasonably mirrors that of a malignant lesion since definitive diagnosis is exclusively histological.
Case Presentation
A 66-year-old men with a history of compensated alcoholic liver disease, emphysema, chronic obstructive pulmonary disease, irritable bowel syndrome and hypertension was referred for the management of a cecal mass.
The patient presented with abdominal pain and chronic intermittent diarrhea worsening over a period of 3 weeks. Diagnostic evaluation was negative for infectious work-up.
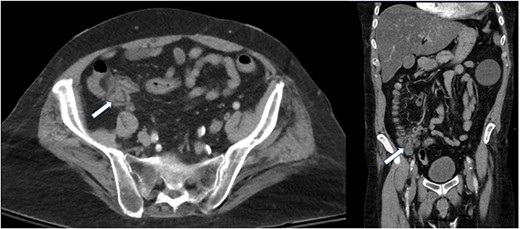
A computed tomography of the abdomen and pelvis (CT A/P) revealed a lobulated 2.4 × 2 cm mass at the cecal base without ascites or obvious metastatic disease (Fig. 1).
CT chest showed a 1.8 × 1.5 cm left lower lung opacity consistent with atelectasis or pneumonia, but malignancy could not be ruled out.
The patient’s complete blood cell count and comprehensive metabolic panel were unremarkable. Further work-up revealed an elevated ferritin consistent with iron overload attributed to chronic liver disease. Tumor markers were mildly elevated (Table 1).
Alfa fetoprotein, prostate specific antigen and carbohydrate antigen 19-9 levels were within normal limits. In the presence of a lung nodule and mildly elevated tumor markers, a PET-CT was therefore done to rule out disseminated disease. A mildly hypermetabolic left lower lung nodule and a hypermetabolic mass contiguous with the appendix and cecum were confirmed.
Further evaluation with colonoscopy showed multiple polyps in the ascending, descending and sigmoid colon without a definite cecal mass. The appendiceal orifice was patent (Fig. 2). Histopathology of the polyps was consistent with multiple tubular adenomas without dysplasia, granulomas or invasive carcinoma.
| . | . | Normal range . |
|---|---|---|
| Carcinoembryonic antigen | 10.8 ng/ml | 0–4.7 ng/ml |
| Chromogranin A | 12 nmol/l | 0–5 nmol/l |
| Ferritin | 904 ng/ml | 30–400 ng/ml |
| . | . | Normal range . |
|---|---|---|
| Carcinoembryonic antigen | 10.8 ng/ml | 0–4.7 ng/ml |
| Chromogranin A | 12 nmol/l | 0–5 nmol/l |
| Ferritin | 904 ng/ml | 30–400 ng/ml |
| . | . | Normal range . |
|---|---|---|
| Carcinoembryonic antigen | 10.8 ng/ml | 0–4.7 ng/ml |
| Chromogranin A | 12 nmol/l | 0–5 nmol/l |
| Ferritin | 904 ng/ml | 30–400 ng/ml |
| . | . | Normal range . |
|---|---|---|
| Carcinoembryonic antigen | 10.8 ng/ml | 0–4.7 ng/ml |
| Chromogranin A | 12 nmol/l | 0–5 nmol/l |
| Ferritin | 904 ng/ml | 30–400 ng/ml |
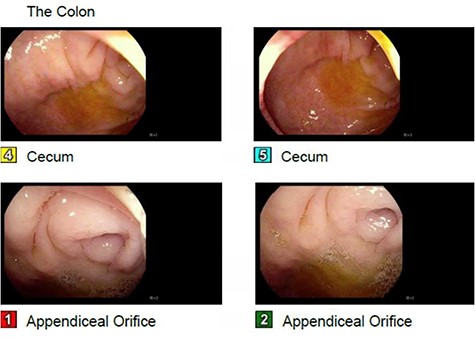
Absence of a cecal mass on colonoscopy, patent appendiceal orifice.
The patient underwent a laparoscopic right hemicolectomy after deliberation of his case at our multidisciplinary tumor board. Intraoperatively, the appendix was not clearly visualized but there was a firm inflammatory mass at the cecal base.
Histological sections demonstrated a nodular inflammatory mass at the appendiceal base with associated serositis. The lesion had an infiltrate of macrophages with Michaelis–Gutmann bodies highlighted by Von Kossa stain (Fig. 3). Once sectioned, a 0.3-cm tan sessile polyp was noted 1.7 cm from the appendiceal orifice. The appendix was dilated, and the lumen filled with mucinous fluid. There was no dysplasia or carcinoma. Fifteen harvested lymph nodes were benign.
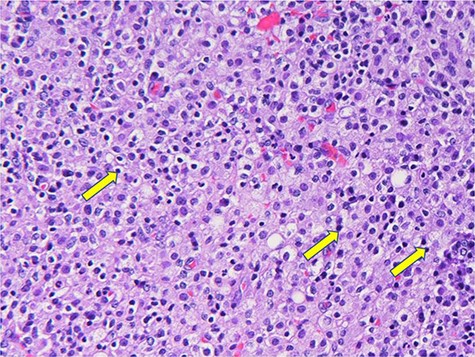
Histiocytes with basophilic Michaelis–Gutman bodies (yellow arrows).
Discussion
Malakoplakia is a benign granulomatous inflammation characterized by an infiltrate of von Hansenmann cells, eosinophilic granular macrophages with basophilic intracytoplasmic inclusions (Michaelis–Gutmann bodies) on histopathology [1]. This benign condition is thought to result from defective phagolysomal activity [2]. Electron microscopy of Michaelis–Gutmann bodies exhibits partially digested bacteria. Immunohistochemistry is consistent with histiocytes.
The pathogenesis of malakoplakia is poorly understood. However, chronic inflammation in the setting of defective phagocytic activity has been proposed. Cutaneous, gastrointestinal and most commonly urogenital malakoplakia is most commonly reported in association with an immunocompromised state. Malakoplakia can also occur with infectious, inflammatory or neoplastic processes. Clinical presentation varies from microscopic disease to plaques, nodules, polypoid lesions and small masses.
After an exhaustive literature search, we were unable to locate similar case reports in which appendiceal malakoplakia presented as a cecal mass. We therefore can only speculate as to the etiology of this mass and its microscopic signature. There is a possibility that chronic obstruction of the appendix from the sub-centimeter polyp noted on pathology led to a sustained localized inflammatory response. Conceptually, this culminated into an inflammatory cecal mass.
Defective phagocytic activity has been cited in association with chronic liver disease [3]. We postulate that our patient’s alcoholic liver disease might have been a driving factor in him having von Hansenmann cells. In addition, high plasma ferritin levels seen with chronic liver disease or sustained inflammation have been associated with immunosuppression. Ineffective phagocytosis has been documented in iron-overloaded patients as compared with normal controls. Macrophages were shown to have decreased phagocytosis of opsonized staphylococcus albicans [4]. Our patient’s ferritin was elevated, and he had chronic diarrhea. It is thus conceivable that his macrophage system had defective phagocytic and bactericidal activity which therefore led to the pathognomonic MG bodies that are remnants of mineralized phagosomes with partially digested bacteria.
Even though final pathology was benign, surveillance poses a dilemma since malakoplakia is associated with a myriad of neoplastic and benign conditions. In the gastrointestinal tract, this entity is reported to be highly associated with malignancy [1]. The presence of a dominant mass should thus encourage patient follow-up.
Conclusions
Appendiceal malakoplakia presenting as a cecal mass is extremely uncommon. The diagnostic evaluation and treatment of a dominant mass reasonably mirrors that of a neoplastic lesion. However, post-treatment surveillance of malakoplakia without malignancy or dysplasia is a surgeon’s conundrum since the pathogenesis and clinical significance of this entity is poorly understood.
Currently, there are no guidelines delineating post-treatment surveillance. However, cautiously assigning malakoplakia as a harbinger of malignancy or a precursor lesion especially in the presence of a dominant mass is reasonable. Management and future directions will potentially be drawn from longitudinal follow-up of these patients.
Conflict of interest statement
None.



