-
PDF
- Split View
-
Views
-
Cite
Cite
Dawit Ayalew, Yahya Alwatari, Lin M Riccio, Massive megarectum secondary to constipation in institutionalized patient, Journal of Surgical Case Reports, Volume 2020, Issue 3, March 2020, rjaa047, https://doi.org/10.1093/jscr/rjaa047
Close - Share Icon Share
Abstract
Chronic constipation is a common cause of morbidity in the elderly and institutionalized population. It can be associated with significant morbidity and socioeconomical burden. Chronic resistance constipation can rarely be associated with megarectum. Herein, we present the case of a patient with physical and mental disability that presented with refractory constipation associated with extreme stool burden and a massive megarectum. We discuss chronic constipation in the elderly population, its etiologies and diagnostic work-up including surgical options. The management of chronic constipation with megarectum should be on a case-by-case basis.
INTRODUCTION
Constipation is one of the leading chronic diseases affecting the elderly. The WHO projects >50% increase in the elderly population (>60 years) in the next 30 years with management of constipation being an anticipated global economic burden [1]. Currently, 16–20% of the general population suffers from constipation with a prevalence as high as 33.5% in the elderly [2]. Within this latter population, institutionalized patients have 48–62% higher prevalence of constipation and 74% are dependent upon daily laxative use [3]. It is estimated that females are two to three times more likely to suffer from severe constipation [3,4]. Chronic constipation has significant physical and psychological consequences in patients’ quality of life. In extreme cases, it can lead to stercoral ulceration and colonic perforation. Constipation is a significant burden on health care resources and is the source of 2.5 million provider visits and 100 000 hospital admissions [5]. Management of refractory constipation in elderly can be challenging. We present a case of massive megarectum in an institutionalized patient secondary to prolonged constipation, including a discussion of diagnostic and management options.
CASE REPORT
A 64-year-old female who resides in a nursing facility due to significant mental and physical disability presented to the emergency department with a 4-month history of chronic intermittent constipation. She had been treated multiple times for severe constipation and fecal impaction. On presentation, abdominal X-rays exhibited massive stool burden filling the rectum, which was distended cephalad to the diaphragm and anteriorly to the peritoneum occupying much of the abdominal cavity (Fig. 1). The patient was admitted to the hospital and aggressive bowel regimen was initiated with limited improvement. Given a history of multiple failed attempts with pharmacological therapy, surgical intervention was considered. A computed tomography scan of the abdomen showed a massive amount of stool in the rectosigmoid colon with no evidence of an obstructing lesion. The patient had undergone colonoscopy within 2 years of presentation with no evidence of polyps or malignant process. She was chronically malnourished, bedbound, non-communicative putting her at significant risk of morbidity with major intervention. Risks and benefits were discussed with the patient’s family, and it was opted for the least invasive surgical approach. We proceeded with creation of a loop colostomy of the descending colon through a mini left to midline 2.5 cm incision. The patient tolerated the procedure well. Post-operatively, she was treated with antegrade enemas through the distal limb of the colostomy as well as enemas and suppositories per rectum. Subsequent X-rays showed significant decrease in stool burden over the 14-Day period (Fig. 2). She was ultimately discharged to her nursing facility with normal bowel function.
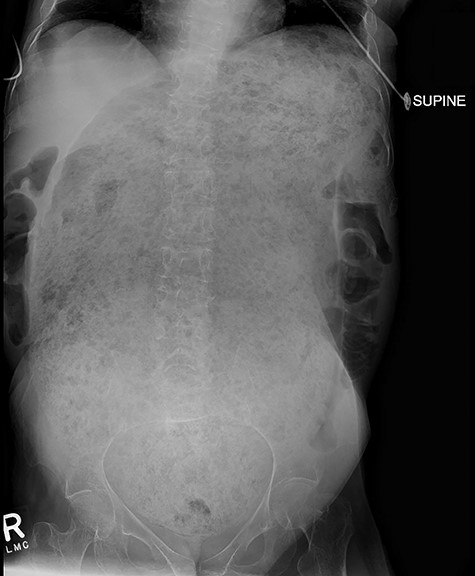
Demonstrates massive megarectum secondary to large stool burden in context of chronic constipation.
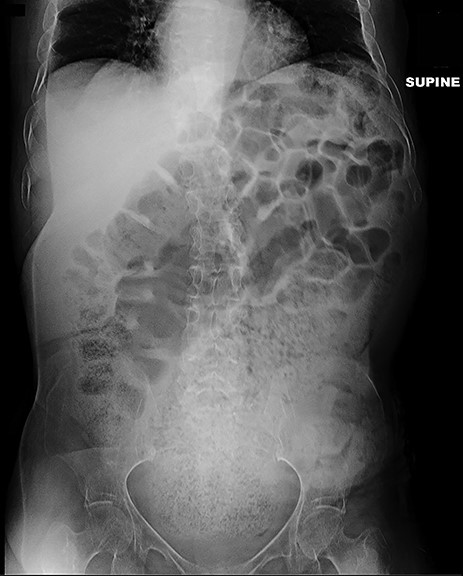
Plain film 2 weeks post-operatively shows significant reduction in stool burden and rectal dilation.
DISCUSSION
It is important to standardize the definition and severity of constipation in order to guide appropriate management. Constipation can be identified per the Rome criteria [Table 1] and severity can be estimated using the Bristol Stool (BSS) and Wexner Constipation Scores (WCS). The BSS uses a picture model while WCS uses a multifactorial approach to grading eight variables of defecation [6].
| ROME III and IV diagnostic criteria . |
|---|
| Functional constipation |
| Must include two or more of the following: |
| – Straining during > 25% of defecations |
| – Lumpy or hard stools (BSS scale 1 or 2) > 25% of defecations |
| – Sensation of incomplete evacuation > 25% of defecations |
| – Sensation of anorectal obstruction/blockage > 25% of defecations |
| – Manual maneuvers to facilitate > 25% of defecations (such as digital evacuation, or support of the pelvic floor) |
| – Fewer than 3 spontaneous bowel movements per week |
| IBS [Rome IV] |
| Recurrent abdominal pain, occurring on average, at least 1 Day per week in the last 3 months, and associated with two or more of the following: |
| – Defecation |
| – A change in frequency of stool |
| – A change in form (appearance) of stool |
| ROME III and IV diagnostic criteria . |
|---|
| Functional constipation |
| Must include two or more of the following: |
| – Straining during > 25% of defecations |
| – Lumpy or hard stools (BSS scale 1 or 2) > 25% of defecations |
| – Sensation of incomplete evacuation > 25% of defecations |
| – Sensation of anorectal obstruction/blockage > 25% of defecations |
| – Manual maneuvers to facilitate > 25% of defecations (such as digital evacuation, or support of the pelvic floor) |
| – Fewer than 3 spontaneous bowel movements per week |
| IBS [Rome IV] |
| Recurrent abdominal pain, occurring on average, at least 1 Day per week in the last 3 months, and associated with two or more of the following: |
| – Defecation |
| – A change in frequency of stool |
| – A change in form (appearance) of stool |
| ROME III and IV diagnostic criteria . |
|---|
| Functional constipation |
| Must include two or more of the following: |
| – Straining during > 25% of defecations |
| – Lumpy or hard stools (BSS scale 1 or 2) > 25% of defecations |
| – Sensation of incomplete evacuation > 25% of defecations |
| – Sensation of anorectal obstruction/blockage > 25% of defecations |
| – Manual maneuvers to facilitate > 25% of defecations (such as digital evacuation, or support of the pelvic floor) |
| – Fewer than 3 spontaneous bowel movements per week |
| IBS [Rome IV] |
| Recurrent abdominal pain, occurring on average, at least 1 Day per week in the last 3 months, and associated with two or more of the following: |
| – Defecation |
| – A change in frequency of stool |
| – A change in form (appearance) of stool |
| ROME III and IV diagnostic criteria . |
|---|
| Functional constipation |
| Must include two or more of the following: |
| – Straining during > 25% of defecations |
| – Lumpy or hard stools (BSS scale 1 or 2) > 25% of defecations |
| – Sensation of incomplete evacuation > 25% of defecations |
| – Sensation of anorectal obstruction/blockage > 25% of defecations |
| – Manual maneuvers to facilitate > 25% of defecations (such as digital evacuation, or support of the pelvic floor) |
| – Fewer than 3 spontaneous bowel movements per week |
| IBS [Rome IV] |
| Recurrent abdominal pain, occurring on average, at least 1 Day per week in the last 3 months, and associated with two or more of the following: |
| – Defecation |
| – A change in frequency of stool |
| – A change in form (appearance) of stool |
Chronic constipation can be of primary or secondary cause. Primary constipation is a direct result of dysfunction in colonic regulation of stool movement which can arise from incoordination of the anorectal neuromuscular system or dysfunction of the brain-gut axis [7]. It can be subdivided as slow transit or as dyssynergia of abdominal and anorectal muscles [7,8]. Secondary constipation results from anatomical blockage, low fiber diet, metabolic disorders or medications side-effects. It is important to recognize that constipation can be a symptom of systemic diseases, such as inflammatory bowel syndrome (IBS). Idiopathic constipation with recurrent fecal impaction can lead to a grossly dilated megarectum [9].
The assessment of chronic constipation includes a detailed history including length of time and straining, maneuvers/medications needed to assist in defection and stool consistency [8]. Abdominal and digital rectal exam is important to assess for masses and strictures. In the absence of gross external abnormalities and secondary causes of constipation, several diagnostic tests can be considered [8]. These include: magnetic resonance, barium defecography (to assess for rectocele, rectal prolapse or intussusception), anorectal manometry and balloon expulsion test (to assess for dyssynergia defecation) [9]. Colonic transit can be assessed with radio-opaque markers, scintigraphy and wireless motility capsule. Colonic manometry can also be used to measure overall colonic motor function. The aforementioned tests provide useful information but have limitations including poor agreement between observers, patient discomfort and costs. Our patient suffered from severe mental and physical disability with chronic malnutrition. These comorbidities compounded by the inability to communicate pain or participate in exam significantly affected her work-up. Furthermore, the size of rectal dilation limited the diagnostic investigations including nuclear tracing and defecography. Secondary functional constipation due to bedridden status was presumed to be the most likely diagnosis, however, differential diagnoses are broad, including adult onset Hirschsprung’s disease and delayed presentation of colic dysmotility.
Multiple surgical options can be considered for management of refractory constipation including subtotal colectomy +/− ileorectal anastomosis, segmental resections, cecostomy tube and more recently sacral nerve modulation (Table 2) [10]. Given the patient’s overall condition and limited interventional options, we believed that proceeding with colonic diversion through mini incision was the most feasible approach. Laparoscopic diversion was not considered due to increased risk of iatrogenic perforation and limited space within the abdominal cavity due to massive, stool-filled rectum. The patient underwent successful creation of loop colostomy without difficulty or complications. Post-operatively, the patient was placed on an aggressive bowel regimen that included mineral oil and soap suds enemas, both antegrade down the distal limb of the colostomy as well as retrograde per rectum. As a result, 20 pounds of stool was eventually evacuated. The patient returned to her regular diet and she was ultimately discharged to her skilled nursing facility in stable condition with a functioning colostomy. It is imperative to address chronic constipation in a timely manner and be cognizant of this prevalent condition in the institutionalized and/or chronically ill elderly patient population. Surgical management should be tailored to each individual’s comorbidities, constipation etiology and response to medical interventions.
| Procedure . | Indications . | Complications . |
|---|---|---|
| Colonic resection | – Evidence of slow transit constipation | – Risk of adhesive disease, recurrent small bowel obstruction |
| – Anastomosis related complication | ||
| Rectal suspension | – High grade intussusception | – Recurrent UTIs |
| – Solitary rectal ulcer syndrome | – Small bowel obstruction | |
| – Rectocele | – Mesh complications | |
| Rectal excision | – Evidence of treatment-resistant obstructed defection syndrome | – Post-operative bleeding, sepsis |
| – Rectocele | – Anastomotic dehiscence | |
| – Rectal stenosis | ||
| – Chronic anorectal pain | ||
| Rectovaginal reinforcement | – Functioning, >3 cm sized rectocele | – Post-operative bleeding |
| – Dyspareunia | ||
| Sacral nerve stimulation | – Medication-resistant constipation | – Infection |
| – Treatment resistance necessitating removal of device |
| Procedure . | Indications . | Complications . |
|---|---|---|
| Colonic resection | – Evidence of slow transit constipation | – Risk of adhesive disease, recurrent small bowel obstruction |
| – Anastomosis related complication | ||
| Rectal suspension | – High grade intussusception | – Recurrent UTIs |
| – Solitary rectal ulcer syndrome | – Small bowel obstruction | |
| – Rectocele | – Mesh complications | |
| Rectal excision | – Evidence of treatment-resistant obstructed defection syndrome | – Post-operative bleeding, sepsis |
| – Rectocele | – Anastomotic dehiscence | |
| – Rectal stenosis | ||
| – Chronic anorectal pain | ||
| Rectovaginal reinforcement | – Functioning, >3 cm sized rectocele | – Post-operative bleeding |
| – Dyspareunia | ||
| Sacral nerve stimulation | – Medication-resistant constipation | – Infection |
| – Treatment resistance necessitating removal of device |
| Procedure . | Indications . | Complications . |
|---|---|---|
| Colonic resection | – Evidence of slow transit constipation | – Risk of adhesive disease, recurrent small bowel obstruction |
| – Anastomosis related complication | ||
| Rectal suspension | – High grade intussusception | – Recurrent UTIs |
| – Solitary rectal ulcer syndrome | – Small bowel obstruction | |
| – Rectocele | – Mesh complications | |
| Rectal excision | – Evidence of treatment-resistant obstructed defection syndrome | – Post-operative bleeding, sepsis |
| – Rectocele | – Anastomotic dehiscence | |
| – Rectal stenosis | ||
| – Chronic anorectal pain | ||
| Rectovaginal reinforcement | – Functioning, >3 cm sized rectocele | – Post-operative bleeding |
| – Dyspareunia | ||
| Sacral nerve stimulation | – Medication-resistant constipation | – Infection |
| – Treatment resistance necessitating removal of device |
| Procedure . | Indications . | Complications . |
|---|---|---|
| Colonic resection | – Evidence of slow transit constipation | – Risk of adhesive disease, recurrent small bowel obstruction |
| – Anastomosis related complication | ||
| Rectal suspension | – High grade intussusception | – Recurrent UTIs |
| – Solitary rectal ulcer syndrome | – Small bowel obstruction | |
| – Rectocele | – Mesh complications | |
| Rectal excision | – Evidence of treatment-resistant obstructed defection syndrome | – Post-operative bleeding, sepsis |
| – Rectocele | – Anastomotic dehiscence | |
| – Rectal stenosis | ||
| – Chronic anorectal pain | ||
| Rectovaginal reinforcement | – Functioning, >3 cm sized rectocele | – Post-operative bleeding |
| – Dyspareunia | ||
| Sacral nerve stimulation | – Medication-resistant constipation | – Infection |
| – Treatment resistance necessitating removal of device |
Funding
There was no funding approved for this project.
Conflict of interest statement
None declared.



