-
PDF
- Split View
-
Views
-
Cite
Cite
Yuta Koichi, Hiroto Kitahara, Naohiro Wakabayashi, Hayato Ise, Chiharu Tanaka, Sentaro Nakanishi, Natsuya Ishikawa, Hiroyuki Kamiya, Pulmonary artery banding for initial treatment of ventricular septal rupture, Journal of Surgical Case Reports, Volume 2020, Issue 3, March 2020, rjaa010, https://doi.org/10.1093/jscr/rjaa010
Close - Share Icon Share
Abstract
Ventricular septal rupture (VSR) is a serious and fatal mechanical complication after acute myocardial infarction. Emergent or urgent, surgical/transcatheter intervention is necessary to treat VSR, though the outcome is not favorable. We performed temporary pulmonary artery banding (PAB) in an 85-year-old man who presented with chest pain to adjust the shunt flow through the VSR, which prevented further pulmonary edema and delayed the timing of surgical repair. There has been no report showing successful PAB performed for VSR after myocardial infarction.
Introduction
Ventricular septal rupture (VSR) is a serious and fatal mechanical complication after acute myocardial infarction [1]. The preoperative status is known as a significant risk factor for morbidity and mortality after surgical VSR closure [2]. Either surgical or transcatheter intervention needs to be performed emergently to prevent hemodynamic collapse; however, it has been reported that early surgical repair had worse outcomes than late surgical repair [3]. In acute myocardial infarction, it is always challenging to perform an intervention, either patch or transcatheter closure, at an early stage because of the friable necrotic tissue. There has been no report of successful pulmonary artery banding (PAB) performed for VSR after myocardial infarction. We successfully stabilized the preoperative hemodynamic status of a patient by attempting PAB and performed the repair procedure at a later stage.
Case Report
An 85-year-old man presented with a complaint of chest pain. Electrocardiogram displayed complete right bundle branch block with ST segment elevation in leads V1 through V4. In addition, echocardiography revealed a reduced left ventricular ejection fraction of 40% and asynergy of the apical wall. Coronary angiography showed occlusion of the middle segment of the left anterior descending (LAD) artery and severe stenosis of the left circumflex artery. He was diagnosed with acute myocardial infarction, and percutaneous coronary intervention was performed for the occlusion of the middle segment of the LAD, achieving revascularization.
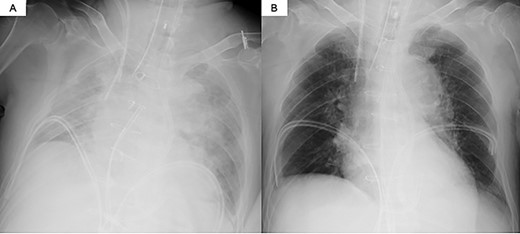
Ten days after the event, he suffered from dyspnea on exertion, and the chest radiography showed massive pulmonary edema. Echocardiography revealed a reduced left ventricular ejection fraction of 30% and a left-to-right shunt in the apical region of the interventricular septum. He was diagnosed with VSR and required inotropic therapy (dopamine 3 μg/kg/min, dobutamine 3 μg/kg/min, adrenaline 0.1 μg/kg/min and noradrenaline 0.1 μg/kg/min) to maintain end-organ perfusion. Given his age and critical condition, surgical VSR closure as an early repair was considered a high-risk procedure.
The decision was made to proceed first with PAB to stabilize the hemodynamics and delay the timing of surgical repair. After median sternotomy, PAB was attempted with a 4-mm wide Teflon felt band. The size of the band was adjusted to where the left to right shunt disappeared under transesophageal echocardiographic guidance (Fig. 1). Postoperative echocardiography revealed a bidirectional shunt through the VSR (Qp/Qs = 1.3), and the chest radiography showed decreased pulmonary congestion (Fig. 2). Cardiac output was maintained to allow adequate end-organ perfusion after PAB (urinary output >100 ml/h). Surgical VSR closure with the infarct exclusion technique and coronary artery bypass grafting were performed 7 days after PAB. The PAB was released after the initiation of cardiopulmonary bypass. Through the infarction area of the left ventricle, the right and left sides of the VSR and infarcted area were approached and covered with a bovine pericardial patch. No residual left to right shunt was observed on transesophageal echocardiography. On postoperative Day 32, the patient was discharged to a rehabilitation hospital.
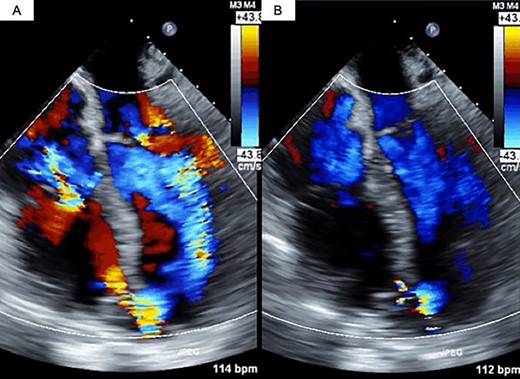
Transesophageal echocardiography findings. (A) Pre-PAB; (B) post-PAB
Discussion
The prevalence of VSR has been reported to be 0.25–0.31% [4]. The clinical presentation of VSR varies from complete hemodynamic instability to frank circulatory collapse depending on the size of the defect, presence of right ventricular infarction and ongoing ischemia, although most patients with VSR are in cardiogenic shock. Along with cardiogenic shock, the defect induces volume overload in the right heart and pulmonary circulation, which can cause pulmonary edema. Either surgical or transcatheter intervention is necessary for VSR, although the optimal timing of the interventions remains controversial.
Daggett et al. [3] reported that the operative mortality rate among patients undergoing surgical repair >21 days after myocardial infarction was 7%. However, if the operation is performed earlier than 21 days, the operative mortality rate is substantially >52%. Lundblad et al. [5] reported that the duration between the onset of VSR and surgical intervention tended to be longer in patients who survived after VSR repair than in non-survivors. Conversely, current guidelines from the American College of Cardiology Foundation and the American Heart Association recommend emergency surgical intervention for VSR [6].
PAB was described as a safe and effective palliative surgical procedure to prevent pulmonary congestion and maintain the cardiac output in patients with ventricular septal defect [7]. Further, PAB has been reported to be adopted in an adult patient with a congenital heart defect [8]; however, there has been no report of successful PAB performed for VSR after myocardial infarction.
Pulmonary artery catheterization for pulmonary artery flow obstruction has been reported, with effects similar to that of PAB. Babb et al. [9] created an external left-to-right ventricular shunt in five dogs. Inflation of a balloon-tipped catheter in the main pulmonary artery reduced the average Qp/Qs ratio from 2.26 to 1.28. In the clinical setting, Grant et al. [10] reported a case of successful hemodynamic stabilization using pulmonary artery catheterization in a patient with VSR. However, compared to pulmonary artery catheterization, PAB is a more direct and certain maneuver that aids in the control of the shunt flow.
In our case, PAB successfully reduced left-to-right shunt flow, prevented further pulmonary congestion and delayed the surgical repair. Accumulation of further similar cases is necessary to establish the effectiveness and safety of PAB as a surgical intervention for VSR.



