-
PDF
- Split View
-
Views
-
Cite
Cite
Katrina Tulla, Shannon Caesar-Peterson, Anouchka Coste, Joye Wang, Norman Morrison, Rare presentation of sepsis caused by necrotizing scalp infection, Journal of Surgical Case Reports, Volume 2020, Issue 3, March 2020, rjaa024, https://doi.org/10.1093/jscr/rjaa024
Close - Share Icon Share
Abstract
Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) is a rising cause of skin and soft tissue infections over the last decade with potentially serious complications. In this article, we describe a case of a large scalp and post-auricular abscess complicated by bacteremia. This is a case of a 73-year-old female who presented with altered mental status was found to have two fluctuant scalp abscesses, bacteremia with necrosis. The patient was promptly treated with intravenous antibiotics, multiple operative debridements without calvarial periosteum involvement defects requiring split-thickness skin grafts for wound closure. This case highlights the severity of a CA-MRSA skin infection in an atypical location.
INTRODUCTION
First described in 1961, methicillin-resistant Staphylococcus aureus (MRSA) is the most common cause of abscesses in the USA [1, 2]. MRSA can be categorized as either hospital-acquired (HA-MRSA) or community-acquired (CA-MRSA), with HA-MRSA being historically more prevalent. However, in the last decade, the incidence of CA-MRSA has increased by over 800% in some parts of the U.S. [2]. Skin and soft tissue infections (SSTIs) caused by CA-MRSA are usually limited to furunculosis and cellulitis, but can also cause more aggressive sequelae than those caused by HA-MRSA [3]. According to Moran et al. [1], the areas affected by MRSA from most to least common include the extremities, torso, perineum, head and neck. Another study demonstrated amongst the CA-MRSA positive head and neck abscesses that require incision and drainage, the scalp was only affected in 4% of the cases, with the face being the most common at 45% [4]. Here, we present a unique case of severe scalp MRSA cellulitis complicated by abscesses and bacteremia, requiring extensive salvage resection, multiple debridements and skin-graft repair.
CASE REPORTS
A 73-year-old female with hypertension, syphilis and type 2 diabetes mellitus was brought in by her daughter for acute altered mental status. Notably, 1 month ago she experienced a mechanical fall, and subsequently developed a pruritic scalp and purulent drainage from sinuses on the scalp behind her right ear. Her vital signs on day of presentation were temperature of 97.9°F, heart rate of 127 bpm, respiratory rate of 22 breaths/min, blood pressure of 144/80 mmHg, with an oxygen saturation of 98% on room air. On physical examination, the patient was oriented to person and place, but not to time. The patient’s face, scalp and right ear were erythematous and edematous. There was a large fluctuant mass with purulent drainage over the occipital and parietal portions of the scalp, with a posterior auricular mass without signs of active drainage. Both of these findings were concerning for underlying abscesses. Her labs showed hyperglycemia to 700, an anion gap metabolic acidosis with serum bicarbonate of 20 and ketonuria. A non-contrast head computed tomography (CT) showed extensive multifocal scalp swelling along the right temporal, right posterior parietal and left frontal regions with no acute intracranial abnormalities. She was admitted to the intensive care unit for diabetic ketoacidosis and cellulitis of the scalp with concern for underlying abscesses. The patient was placed on a continuous infusion of insulin with broad-spectrum coverage with intravenous Vancomycin and Cefepime. She subsequently underwent incision and drainage of scalp lesion on hospital day 2, revealing a 10-cm subgaleal abscess with large amounts of purulence evacuated. On hospital day 3, initial admission blood cultures grew MRSA. The patient was continued on intravenous Vancomycin and Cefepime. A paranasal sinus CT with intravenous contrast did not show involvement. The patient also underwent a transesophageal echocardiogram without evidence for endocarditis. Patient returned to the operating room on hospital days 4 and 5 for further pulse irrigation of the wounds and sharp debridement with the involvement of plastic surgery and continued to have serial bedside debridements (Fig. 1). The scalp wound ultimately measured 20 cm in length, 10 cm in width and 2 cm depth. The right posterior auricular wound measured 7 × 7 × 2 cm. The wounds of the scalp and post-auricular region were definitively closed with split-thickness skin grafts harvested from the right thigh (Fig. 2). There was 100% take of the grafts on postoperative day 7. The patient was discharged on hospital day 32 on intravenous Vancomycin to complete a full 7-week course (Fig. 3).
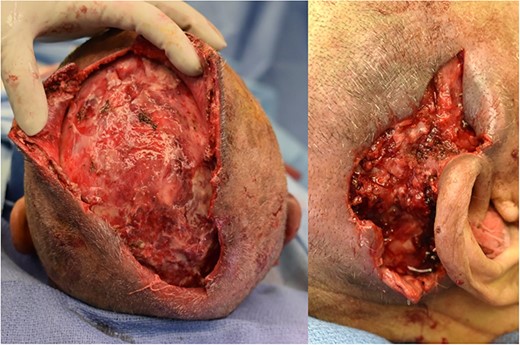
Wound bed of scalp (left) and posterior auricular (right) wounds after multiple debridments. The scalp wound ultimately measured 20 cm in length, 10 cm in width and 2 cm depth. The right posterior auricular wound measured 7 × 7 × 2 cm. Wound bed free of purulent drainage and WBC down trending with sepsis resolving.
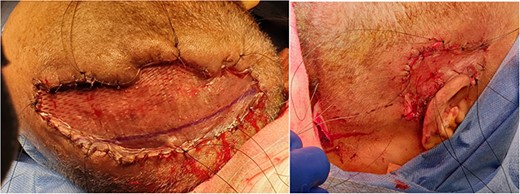
Split-thickness skin graft placement on scalp (left) and right posterior auricular region (right). Split-thickness skin graft placed after multiple bedside and mechanical debridement carried out. Versajet used to clean the wound bed prior to placement of skin graft. Good granulation tissue present and spilt thickness skin graft taken from left thigh and meshed with 1:1.5 dermacarrier.
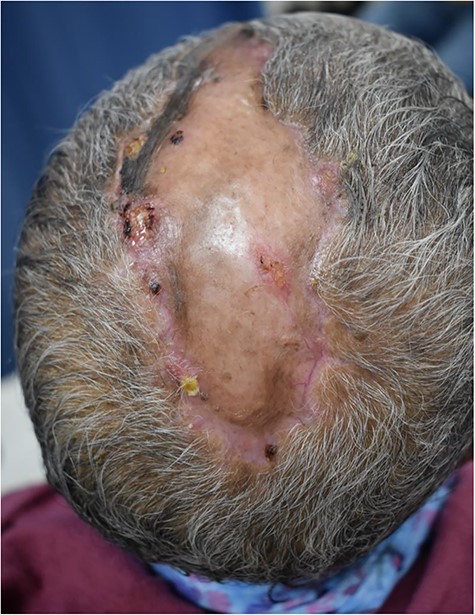
Postoperatively wound after discharge home. Skin graft healed well with some surrounding areas of hyperpigmentation. No areas of the graft were rejected.
DISCUSSION
CA-MRSA is usually found in healthy individuals with a predominance in athletes, incarcerated individuals, children < 2 years old, Native Americans and military recruits [5]. Some CA-MRSA specific risk factors include IV drug use, antibiotic use and comorbidities, such as cancer, chronic skin diseases and diabetes [6, 7]. The pathogenesis of abscess formation and necrosis is thought to be due to the presence of the MRSA-specific panton-valentine leukocidin toxins, a membrane protein that causes dermal necrosis and white blood cell lysis via leukocyte interaction [5]. In this case, the route of inoculation is most likely through the initial injury sustained during her mechanical fall 1 month prior to presentation. Interestingly, the location, severity of her abscesses and the degree of debridement required has not been previously reported in the adult population. In a retrospective analysis done on MRSA abscess incidence in NYC, the scalp only represent 4% of all head and neck cases [4]. Despite adequate antibiotic coverage and surgical drainage, the patient required three additional debridement procedures to achieve adequate source control. Furthermore, 25% of the patient’s scalp was affected including involvement of the galea aponeurotica, necessitating a skin-graft repair. Current guidelines for the management of MRSA SSTIs focus on appropriate antibiotics coverage with incision, drainage for purulent infections with clinic follow-up.
We presented a case of CA-MRSA scalp abscess and bacteremia treated with intravenous antibiotics, incision and drainage and multiple salvage resections, ultimately requiring scalp reconstruction. This case highlights the severity of a CA-MRSA SSTIs in an atypical location requiring multiple extensive debridements and ultimately a skin graft. Early diagnosis and judicious surgical interventions can limit the extent of the disease and prevent fatal complications of CA-MRSA infections.



