-
PDF
- Split View
-
Views
-
Cite
Cite
Chairat Burusapat, Naphan Buarabporn, Kittisak Wongchansom, Pongsit Chanapai, Parinya Parinyanut, Surapong Supaporn, Mammary adenoid cystic carcinoma presenting with Ductal carcinoma in situ and axillary lymph node metastasis, Journal of Surgical Case Reports, Volume 2020, Issue 2, February 2020, rjz362, https://doi.org/10.1093/jscr/rjz362
Close - Share Icon Share
Abstract
Mammary adenoid cystic carcinoma (ACC) is extremely rare tumors, comprising <0.1% of all breast cancers. Moreover, lymph node metastasis is <2% of mammary ACC. Here, we report a case of 51-year-old female presented with painful mass on her left breast and left axillary lymph node enlargement. Core needle biopsy revealed invasive ductal carcinoma. Left lumpectomy and axillary lymph nodes dissections were performed. The final pathological report showed triple-negative mammary ACC arising with high grade ductal carcinoma in situ (DCIS) and axillary lymph node metastasis. Immunohistochemistry study is useful in confirming a diagnosis. Given the rarity of this cancer, natural history of disease is still not clearly understood. Complete surgical excision is the mainstay of treatment. To our best knowledge, mammary ACC presenting with DCIS and axillary lymph node metastasis has never been reported and should be considered in the differential diagnosis of breast cancers.
INTRODUCTION
Mammary adenoid cystic carcinoma (ACC) is extremely rare tumors, comprising <0.1% of all breast cancers. Moreover, lymph node metastasis is seen in <2% of mammary ACC [1]. We present a case of 51-year-old female with triple-negative mammary ACC arising with high grade ductal carcinoma in situ (DCIS) and axillary lymph node metastasis. To our best knowledge, this has never been reported.
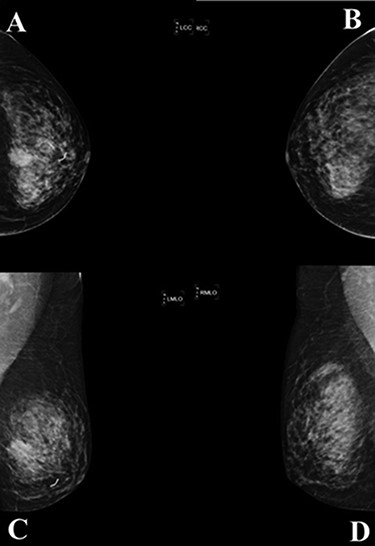
A mammogram of the breasts revealed a mass with angular and ill-defined boarder at palpable area in left mid breast size 23 × 19 mm. (A; left cranial caudal view, C; left mediolateral—oblique view), comparison with the right breast (B; right cranial caudal view, D; right mediolateral—oblique view).
CASE REPORT
A 51-year-old female, with no history of breast surgery, irradiation or pregnancy, presented with mastalgia on her left breast. A physical examination revealed a painful mass measuring 2 × 2 cm on left breast deep to the nipple areolar
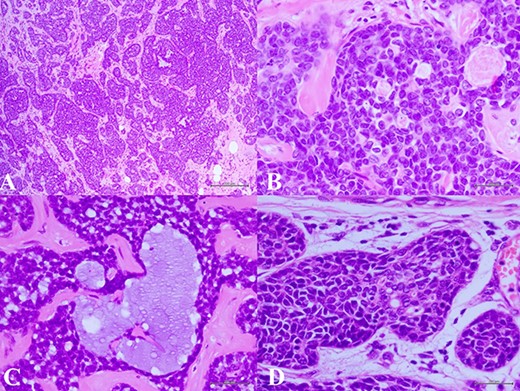
(A) Histologic examination showed the neoplastic cells predominantly arranged in a solid growth pattern (100×). (B) The true glandular spaces lined by luminal-type epithelial cells (600×). (C) The pseudolumens containing watery basophilic mucopolysaccharides basement membrane-like materials rimmed myoepithelial cells (400×). (D) Intraductal components composed of a dual population as the invasive areas (600×)
complex. The left axillary lymph node was found to measure 1 cm after palpation. A mammogram revealed an ill-defined mass at sub-areolar on left breast with angulation 2.3 × 1.9 cm, without suspicious microcalcification or architectural distortion (Fig. 1). Core needle biopsy revealed invasive ductal carcinoma (IDC). Immunohistochemical study (IHC) showed Estrogen receptor (ER), Progesterone receptor (PR), and Human epidermal growth factor receptor 2 (HER2) negative. Fine needle aspiration of left axillary lymph node revealed poorly differentiated metastatic lymph nodes. Metastatic workups were unremarkable. Left lumpectomy and axillary lymph nodes dissection were performed. The final pathology reported ACC measuring 3.5 cm in greatest dimension, lymphovascular invasion, presenting with DCIS, comedonecrosis type, high grade nuclei, microcalcification, all margins are negative for malignancy (Fig. 2). IHC showed CD117 and CK7 positive for malignant ductal cell of tumor, CK5/6 and p63 positive for myoepithelial cells within the tumor. ER, PR and HER-2 were negative. Ki-67 was strongly positive 3 + of nuclear staining, 30% of neoplastic cells (Fig. 3). One of 21 lymph nodes demonstrated metastatic ACC with extranodal extension (Fig. 4). Metastatic part is 0.9 cm in greatest dimension. Postoperative adjuvant chemoradiotherapy was given.
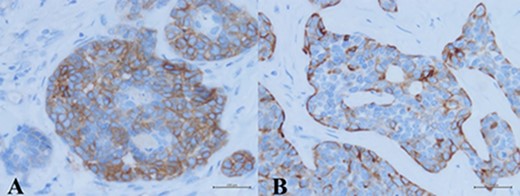
Immunohistochemistry study revealed two different cell populations. The cells surrounding true glandular lumens decorated by CK7 and CD117 (A), while CK5/6 and p63 were positive in myoepithelial cells (B).
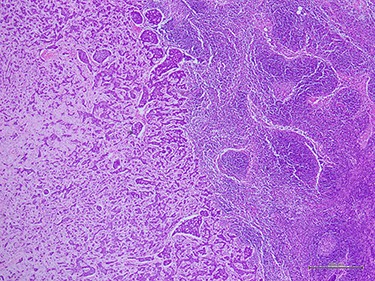
Histologic examination demonstrated metastatic ACC to axillary lymph node (40×).
DISCUSSION
Mammary ACC is a rare neoplasm. Several features distinguish ACC from typical histologic types of breast cancer and suggest that it may have a unique tumor behavior [2]. It is more commonly seen in older female and present as a painful mass [3]. There are no distinctive imaging characteristics for ACC. It can appear as a smooth round mass similar to a benign mass or as an irregular mass on mammogram. Ultrasound demonstrates a hypoechoic or heterogenous mass with minimum vascularity and may have an indistinct margin. No consistent magnetic resonance imaging features have been demonstrated [3, 4].
A cribriform architecture of ACC may mimic cribriform type of IDC and cribriform DCIS, which has led to core biopsy specimens being misdiagnosed [5, 6]. ACC has a unique dual-cell pattern and is indistinguishable from ACC arising from salivary tissue. Diagnostic criteria for ACC require a biphasic cellular pattern comprised of myoepithelial and epithelial cells. Tumor cells were arranged into the glandular-cribriform and cord-like patterns, and the cystic lesion was filled with eosinophilic membrane basement material [7]. IHC to examine basement membrane materials or an ultrastructural examination is recommended to make a correct diagnosis. The basaloid myoepithelial-like tumor cells of ACC strongly expressed p63 and smooth muscle actin and the ductal epithelial cells of ACC were positive for c-kit (CD117). IHC expression of p63 and c-kit distinguishes ACC from invasive cribriform carcinoma and DCIS [5]. Mammary ACC is good prognosis compared with ACC in other locations [8].
ACC has been known to frequently express ER, PR and HER-2 negative, which has been defined as triple-negative breast cancer (TNBC) [3]. The cause and risk factors of ACC are unknown. Previous reports showed ACC arising in fibroadenoma [8], microglandular adenosis [9] and IDC [10]. Our patient demonstrated triple-negative mammary ACC arising with high grade DCIS and axillary lymph node metastasis. Predisposing of ACC from DCIS rarity reported.
While TNBC has been reported to be aggressive than other histologic type, mammary ACC that is often associated with TNBC has been known as a tumor with less progressive potential causing infrequently tumor metastasis or tumor recurrence [7]. Axillary and distant metastases are rare. Distant metastasis may occur without prior lymph node involvement [2, 9].
Given the rarity of this cancer, treatment guidelines are still well established. Complete surgical excision is the mainstay of treatment, but there are not clear guidelines for extent of resection. Surgical treatment options are both mastectomy and breast conserving surgery. Hormonal therapy is not indicated as they are ER/PR negative. The 5-year overall survival rate was 98–100%, and 10-year survival rate ranging from 85 to100% [1]. Follow-up for periods longer than 10 years is recommended. In our patient presented an mammary ACC and axillary lymph node metastasis that rarity presentation. Moreover, ACC arising with high grade DCIS has never been reported. The correlation of ACC and DCIS should be studied in the future.
CONCLUSION
Mammary ACC is extremely rare tumors. Moreover, lymph node metastasis is seen in <2% of mammary ACC. The cause and risk factors of ACC are unknown. Natural history of this disease is still not clearly understood. Recurrence can occur late, and follow-up for periods longer than 10 years is recommended. ACC should be kept in mind in patient with cribriform type of IDC and/or DCIS. IHC is necessary.
Conflict of Interest statement
None declared.



