-
PDF
- Split View
-
Views
-
Cite
Cite
Kailan Sierra-Davidson, Geoffrey Anderson, Kenneth Tanabe, Ozanan R Meireles, Desmoid tumor presenting 2 years after elective Roux-en-Y gastric bypass: a case report and review of the literature, Journal of Surgical Case Reports, Volume 2020, Issue 2, February 2020, rjz379, https://doi.org/10.1093/jscr/rjz379
Close - Share Icon Share
Abstract
Desmoid tumors are rare malignancies derived from myofibroblasts, which can cause significant morbidity due to life-threatening invasion of local structures. Risk factors include familial adenomatous polyposis, antecedent surgical trauma and estrogen exposure. We described a previously healthy 27-year-old female in whom a desmoid tumor developed 2 years after a Roux-en-Y gastric bypass was performed for the treatment of obesity. Computed tomography scan demonstrated a 16-cm complex density intra-abdominal mass. Exploratory laparotomy was performed, revealing a mass firmly adherent to the Roux limb, as well as the jejunojejunostomy and distal portion of the bilopancreatic limb. En bloc resection of the mass and the Roux limb was required, as well as reconstruction of the Roux-en-Y anatomy. This case describes a unique, long-term complication of bariatric surgery, in light of a growing population of patients with altered gastric anatomy.
INTRODUCTION
In the USA, over 36% of adults are obese (defined as body mass index greater than 30 kg/m2). Roux-en-Y gastric bypass (RYGB) remains a common procedure for weight loss with approximately 40 000 operations performed in the USA annually. Long-term studies of bariatric surgery demonstrate sustained excess weight loss of up to 70% 5 years after surgery and improved metabolic health [1].
Desmoid tumors are rare, locally aggressive tumors with low potential for metastases but high rate of recurrence [2]. There is an incidence of two to four per million persons per year, of which approximately 10% are associated with familial adenomatous polyposis (FAP) [3]. Herein is a case report of patient in whom a desmoid tumor developed 2 years after a RYGB was performed for the treatment of obesity.
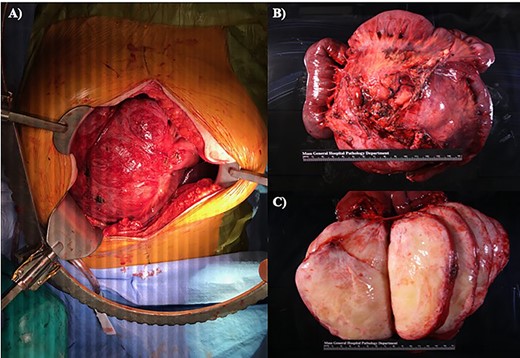
(A) Midline laparotomy with in situ specimen. (B) En bloc resected specimen, consisting of two anastomosing segments of small bowel, as well as a 15.0 × 14.2 × 2.5 cm round mass. (C) Sectioned mass revealing a tan-white, encapsulated gelatinous interior without evidence of necrosis or hemorrhage.
CASE REPORT
A 27-year-old woman with a past medical history of obesity status post RYGB presented to an outside hospital with 1 week of left upper quadrant abdominal pain and an associated palpable mass. She denied nausea, emesis, changes in bowel movements or fevers. There was no family history suggestive of FAP. There was a personal history of two uncomplicated pregnancies. She had an unknown oral contraception history with a progestin-secreting intrauterine device in place. Laboratory analysis revealed non-elevated levels of carcinoembryonic antigen, cancer antigen-125 (CA-125) and alpha-fetoprotein. An abdominal and pelvic computed tomography scan revealed a 16-cm complex density mass. There was no evidence of obstruction of nearby structures. A percutaneous needle biopsy was performed, and pathology was consistent with mesenteric fibromatosis. The patient was planning to become pregnant soon. Due to concerns for increased tumor growth in the setting of pregnancy, the patient opted for resection.
The patient underwent an exploratory laparotomy. The mass was noted to arise from the proximal small bowel and extend from the root of the mesentery to within 1 cm of the bowel wall. The mass extended close to, but did not involve the superior mesenteric artery. The mass was firmly adherent to the Roux limb, as well as the jejunojejunostomy and distal portion of the bilopancreatic limb. The distal small bowel was not involved.
Due to mesenteric involvement of Roux limb, its vascular supply had to be compromised to allow for adequate resection. This caused the entire Roux limb to become ischemic. The resection was extended proximally to include the gastrojejunostomy. The mass was resected en bloc with the entire Roux limb and the jejunojejunostomy. The common channel was divided distal to the jejunojejunostomy, and the biliopancreatic limb was divided approximately 20 cm distal to the Ligament of Treitz. With the specimen removed, the RYGB was reconstructed in an antecolic antegastric fashion. Given the small gastric pouch, an EEA stapler was used to recreate the gastrojejunostomy using the OrVil 25 system. The jejunojejunostomy was then recreated using a linear Endo-GIA white load staple. The patient recovered well from the procedure. She passed a swallow evaluation on post-operative day one and was discharged home on post-operative day four on a liquid diet.
The resected specimen measured 16.5 × 15.5 × 12.5 cm. It consisted of two anastomosing segments of small bowel, as well as a 15.0 × 14.2 × 2.5 cm round mass that was partially adherent to the small bowel serosa in multiple areas (Fig. 1). The mass was sectioned to reveal an encapsulated, white gelatinous interior. The mass was homogeneous without areas of hemorrhage or necrosis. The mass abutted the mesenteric margin and was 4.5 cm from the nearest small bowel margin. Immunohistochemical staining demonstrated beta-catenin expression, consistent with mesenteric fibromatosis. The patient was seen in clinic on post-operative day 15 and has made a full recovery.
DISCUSSION
Desmoid tumors can cause significant morbidity and mortality from obstruction of adjacent structures [4]. Approximately 10% are associated with FAP, where mutations in the ‘adenomatous polyposis coli’ gene cause intracellular accumulation of beta-catenin [2, 3]. Of note, the prevalence of FAP among patients who develop a desmoid tumor without a history of polyps is low (7.5% in one study) [3]. Additional risk factors include pregnancy, exogenous estrogen and antecedent surgical trauma [5, 6].
Diagnosis is centered on imaging, biopsy and immunohistochemistry for beta-catenin, which is expressed in approximately 80% of sporadic cases [2]. Management and treatment is complicated by variable clinical behavior. Although most tumors will grow progressively over time, some have long periods of stability (~50%) or even spontaneous regression (~10%) [2]. Guidelines from the National Comprehensive Cancer Network suggest that monitoring is a reasonable strategy for patients with tumors that are not causing significant impairment [7]. In the case of our patient, surgery was recommended as she was planning to become pregnant. High estrogen levels are thought to drive tumor growth based on retrospective data and tissue histology [5]. Indeed, in small series, 80% of women with pregnancy-associated desmoid tumors who were initially treated conservatively ultimately required resection [5].
Unfortunately, despite surgical excision with negative margins, up to 50% of patients experience recurrence within 5 years [8]. Thus, follow-up imaging is recommended every 3–6 months for 2–3 years, then annually [7].
Given the site of the mass at the anastomosis, the most likely etiology is her prior RYGB. Previous surgical trauma is known risk factor, supporting the biological role of wound-repairing myofibroblasts. In a small series of sporadic cases, prior surgery at the tumor site was found in 28% of patients [9]. Given the role of dysregulated inflammation in the development of these tumors, the contribution of metabolic syndrome requires further investigation.
Although antecedent surgical trauma is an important risk factor for development of desmoid tumors and the frequency of bariatric surgery is increasing, a review of the literature identifies only one other case report of presentation in a patient with bariatric surgery [10]. Of note, this other case report presents a patient following sleeve gastrectomy with less complicated anatomy. Nonetheless, both cases highlight the complicated presentation and surgical resection of desmoid tumors following bariatric surgery.
CONFLICT OF INTEREST STATEMENT
None declared.



