-
PDF
- Split View
-
Views
-
Cite
Cite
Amalan Thuraisingam, An intrathoracic mass behind severe immunodeficiency: a case report of Good’s syndrome and large type AB thymoma, Journal of Surgical Case Reports, Volume 2020, Issue 1, January 2020, rjz394, https://doi.org/10.1093/jscr/rjz394
Close - Share Icon Share
Abstract
A large solitary intrathoracic mass was identified in a patient with concurrent immunodeficiency. The patient was found to have a type AB thymoma and was diagnosed with Good’s syndrome. A case report demonstrates the importance of timely surgical intervention for this rare syndrome.
INTRODUCTION
Good’s syndrome (GS) is a rare form of immunodeficiency that is characterized by the presence of a thymoma with hypogammaglobulinemia [1, 2]. This presentation pattern was first reported by Robert Good in 1954 [1]. It is typically an adult onset of immunodeficiency, usually in the fourth or fifth decade of life [2]. Patients are susceptible to bacterial, viral, fungal and opportunistic infections due to both humeral and cell-mediated immune deficiency [1, 3]. Immunological features of GS include low peripheral B cells and hence hypogammaglobulinemia, CD4+ lymphopenia and reversal of CD4:CD8 ratio [4]. Proposed mechanisms for GS include lack of interferon-like cytokines from thymic T cells to promote B cell growth and differentiation and impaired haemopoiesis from thymoma paraneoplastic phenomena [1,3].
A case report of patient with Good’s syndrome with Type AB thymoma.
CASE REPORT
A 51-year-old female non-smoker presented with several months history of recurrent mouth ulcers, productive cough and chronic diarrhoea. She also experienced constitutional symptoms including fever, night sweats and weight loss. Her past medical history included alopecia and thyroidectomy for multinodular goitre, supplemented with thyroxine. She had been extensively worked up by her LMO and was subsequently found to have a solid left lower thorax mass on CT scan. Her progressively worsening oral ulceration and subsequent weight loss due to odynophagia required an urgent hospital admission.
Her initial blood results revealed leukopenia, low B cell count and hypogammaglobulinaemia: IgG (4.27 g/L, 5.2–16), IgA (0.26 g/L, 0.85–3.50) and IgM (0.23 g/L, 0.32–3.00). Her CD4: CD8 ratio was also consistently low. A preliminary diagnosis was made as a Good’s syndrome in the setting of hypogammaglobulinaemia and presence of an intrathoracic mass. Other differential diagnoses for her ulcerations included paraneoplastic pemphigus and lichen planus. Her negative skin autoantibodies excluded the paraneoplastic pemphigus. Her oral mucosal biopsy revealed a chronic ulcerating lichenoid mucositis. She was commenced on topical theory for her oral lichen planus with dexamethasone. She had a CT-guided biopsy of the left lower thorax mass, which revealed type B thymoma.
During her admission, she developed progressive leukopenia. Her neutrophil count dropped to 0.27 × 109/L with mild thrombocytopenia (143 × 109). A bone marrow biopsy revealed a mildly hyper-cellular bone marrow with evidence of granulocyte maturation block. The marrow was cytogenetically normal, and immunophenotyping of her lymphocytes revealed that this was comprised almost entirely of T cells with virtually absent B cells. She was commenced on intravenous immunoglobulin (IVIg) infusions for immunoglobulin replacement, given significant B-cell deficiency and referred for an urgent inpatient resection of the thymoma.
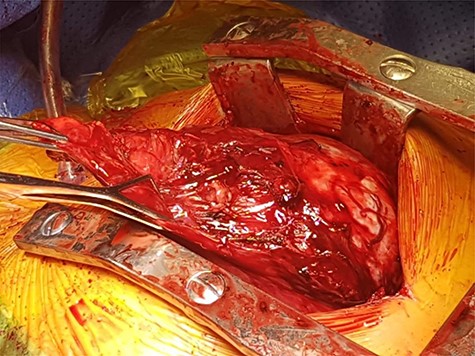
After initial inspection with video assisted thoracotomy, she underwent a median sternotomy and resection of the large thymoma. Intraoperatively, she was found to have a solitary encapsulated solid mass in the left pleural cavity measuring 150 × 130 × 60 mm with a vascular pedicle attached to the anterior mediastinum. After careful dissection of draining vessels and pericardial adhesions, the mass and remaining thymus tissue were excised. The mass was found to weigh approximately 746 g (Figs 1 and 2).
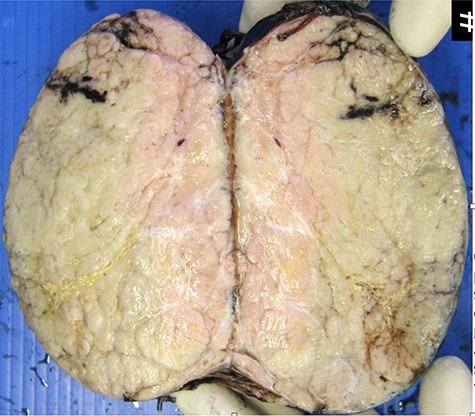
Macroscopic appearance of resected thymoma. Cut section is predominantly solid, homogenous cream in colour, with multilobated appearance separated by thin fibrous septa.
Histology demonstrated both a spindle cell component and a lymphocyte component, consistent with a type AB thymoma, and pathologic staging was pT1a (Fig. 3). Staging CT scan at this time did not reveal any other metastasis. After the successful resection of the thymoma, the patient had ongoing intravenous immunoglobulin (IVIg) infusions. She made a good recovery and was discharged home after a 3-week admission with oncology and immunology follow-up.
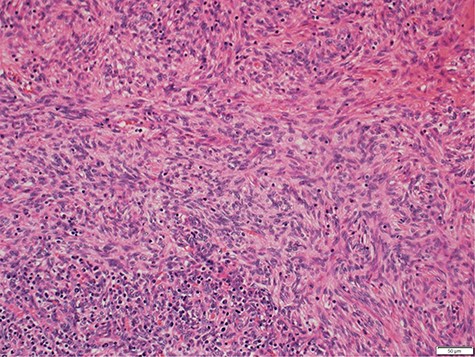
Photomicrograph showing medium magnification of type AB thymoma, composed of spindle cell component and lymphocyte component (biphasic tumour).
At her 3-month review, she remained well with no autoimmune symptoms and recovery of her neutrophil count. Her chronic cough had also improved and was thought to be due to irritation of recurrent laryngeal nerve from her large thymoma. She continued to have her monthly IVIg infusions as her B-cell counts remained low. Interestingly, her T-cell function had remained quantitatively normal throughout her illness despite the fact that Good’s syndrome is often associated with abnormal T cell function. Follow-up CT staging scan did not reveal any recurrence of the mass in the thorax nor any metastasis. Her follow-up plan consists of second monthly review for close monitoring of her lymphocyte count with monthly IVIg infusion as part of her ongoing recovery.
COMMENT
GS has a worse prognosis than other humoral immunodeficiencies [1, 4]. Retrospective case series suggest that GS has survival rates of 82% at 5 years and 68% at 10 years with a median survival of 14 years [4]. Surgical resection of the tumour is indicated; however, removal of the tumour does not always correct the immunodeficiency, and ongoing IVIg may be required [3]. The most important indicator of long-term prognosis is believed to be the completeness of the resection [2].
Meeting Presentation: ANZSCTS, Annual Scientific Meeting, Sofitel Noosa Pacific, Queensland, Australia, 6–11, November 2018.



