-
PDF
- Split View
-
Views
-
Cite
Cite
Masahiro Tsutsui, Hayato Ise, Sentaro Nakanishi, Natsuya Ishikawa, Norifumi Otani, Hiroyuki Kamiya, Dramatic improvement of left ventricular function after switching the ventricular pacing site from the right ventricular apex to the left ventricular free wall via a left mini thoracotomy: a case report, Journal of Surgical Case Reports, Volume 2019, Issue 5, May 2019, rjz155, https://doi.org/10.1093/jscr/rjz155
Close - Share Icon Share
Abstract
The case of a patient with pacemaker-induced cardiomyopathy in whom left ventricular (LV) function was dramatically improved after switching the ventricular pacing site from the right ventricular apex to the LV free wall via a left mini thoracotomy due to pacemaker-associated infective endocarditis (PAIE) is presented. Our experience suggests that a surgically implanted epicardial LV lead on the LV lateral wall can be a good alternative pacing site that preserves LV function, especially in patients with PAIE.
INTRODUCTION
It is well known that right ventricular pacing can cause pacemaker-induced cardiomyopathy (PICM) in approximately 20–50% of patients [1]. The pacing site plays a central role in the development of PICM. The apex of the right ventricle is the worst pacing site and pacing on the ventricular septum can offer more physiological stimulation, avoiding PICM. Once PICM occurs, upgrading to cardiac resynchronization therapy with an additional left ventricular (LV) pacing lead is the therapy of choice [1]. However, very little is known about how LV pacing alone affects LV function, because LV pacing alone is very unusual, and it is normally only done via thoracotomy. The case of a patient with PICM in whom LV function was dramatically improved after switching the ventricular pacing site from the right ventricular apex to the LV free wall via a left mini thoracotomy due to pacemaker-associated infective endocarditis (PAIE) is presented.
CASE REPORT
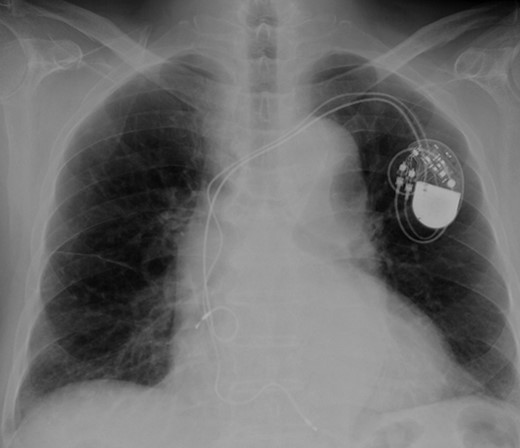
Eight years ago, a DDD pacemaker was implanted in an elderly man due to complete atrioventricular (AV) block. The pacing sites were the appendage of the right atrium and the apex of the right ventricle (Fig. 1) subsequently he suffered PICM with an LV ejection fraction of 37% and obvious dyssynchrony. He had chronic heart failure of NYHA II. Suddenly, the patient developed fever and complained of difficulty eating. Transthoracic echocardiography showed a vegetation (11 × 12 mm2) at the pacemaker leads, and this vegetation was also attached at the tricuspid valve (Fig. 2). Laboratory examination showed elevated white blood cell count and C reactive protein levels. He was diagnosed with PAIE, although the peripheral blood cultures showed no bacteria. After antibiotic therapy, laboratory examination showed decreasing inflammation parameters, but the size of the vegetation remained stable.
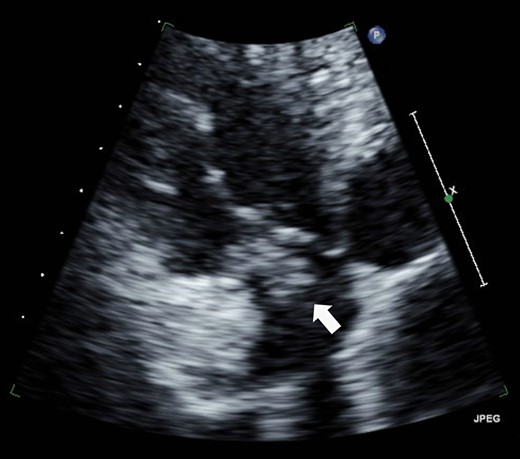
A vegetation on the tricuspid valve (arrow). Transthoracic echocardiography shows a vegetation (11 × 12 mm2) attached at the tricuspid valve.
According to the current guideline for pacemaker infection, it was decided to remove the whole pacemaker system, including the two leads. Since implantation of new transvenous leads seemed undesirable because of ongoing active endocarditis, it was decided to implant epicardial leads on the left atrium and left ventricle via a left mini thoracotomy. Because the patient had complete AV block without any spontaneous R wave, a new DDD pacemaker system was implanted first. The atrial lead was implanted on the appendage of the left atrium, and the ventricular lead was implanted onto the lateral wall of the left ventricle near the first obtuse marginal branch (Fig. 3). The old infected pacemaker system was then explanted by percutaneous extraction.
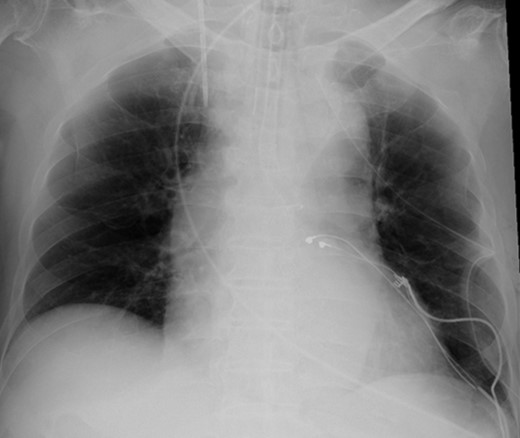
After the operation, the patient recovered rapidly. Of note, postoperative transthoracic echocardiography showed dramatically improved LV function, with EF of 56%. Moreover, dyssynchrony disappeared. On the other hand, the vegetation at the tricuspid valve remained unchanged. He was transferred to a territorial hospital for further therapy with antibiotics on the seventh postoperative day. Now, 3 years after surgery, the patient is doing very well without any sign of heart failure.
DISCUSSION
A case with PAIE in whom the pacemaker system was explanted percutaneously and epicardial leads were implanted via a left mini thoracotomy, resulting in dramatic improvement of LV function, was presented.
In the case of PAIE, it is not always easy to decide how the pacemaker system should be explanted, especially if there is a vegetation along the pacemaker lead on the tricuspid valve. The current guideline recommends that percutaneous methods of lead removal are preferred for infected leads, combined with complete removal of the generator [2]. However, percutaneous extraction of pacing leads is sometimes very dangerous due to adhesions at the brachiocephalic vein or in the heart, and it can result in lethal cardiac tamponade. On the other hand, the guideline also recommends that open surgical removal should be considered for large lead-associated vegetations (>20 mm) and when valve surgery is indicated for other reasons [2], but it needs cardiopulmonary bypass and may not be desirable in very ill patients. In the present case, since the vegetation was 11 × 12 mm2, the percutaneous lead removal method was selected, and the epicardial LV lead was implanted via a left mini thoracotomy to prevent recurrence of PAIE.
Because of the risk of PICM, typically, right ventricular (RV) septal pacing has been preferred to RV apex pacing, and in cases of PICM, cardiac resynchronization therapy (CRT) is the therapy of choice in many cases. In the present case, the epicardial LV lead was surgically implanted, not for treatment of PICM, but to prevent recurrence of PAIE. However, LV dyssynchrony disappeared, and, unexpectedly, LV function improved dramatically.
There have been only a few studies of the effect of the LV lead alone, because it is normally implanted surgically, and, therefore, very unusual as a routine pacing site. Chen et al. reported that using an epicardial LV lead at CRT implantation was equivalent to a coronary sinus approach [3]. Shan et al. reported using an epicardial LV lead for prophylactic use during open heart surgery in patients with low cardiac function likely to require CRT implantation after operation [4]. Bildirici et al. reported that a significant increase in EF was observed in LV pacing group whereas a significant decrease was observed in RV pacing group [5]. However, no one has reported improvement of LV function by changing the pacing site from the right apex of the right ventricle to the epicardial LV lateral wall. To the best of our knowledge, this is the first report of the effect of a surgically implanted epicardial LV lead on LV function, avoiding LV dyssynchrony.
CONCLUSION
Marked improvement in cardiac function using an epicardial LV lead was seen in a patient with PICM and PAIE. This case suggests that a surgically implanted epicardial LV lead on the LV lateral wall can be a good alternative pacing site that preserves LV function, especially in patients with PAIE.
ACKNOWLEDGMENTS
None
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest.



