-
PDF
- Split View
-
Views
-
Cite
Cite
Hasan Dagmura, Emin Daldal, Ahmet akbaş, Fatih Daşıran, A rare anal mass: anal leiomyoma presented as perianal fistula, Journal of Surgical Case Reports, Volume 2019, Issue 2, February 2019, rjy351, https://doi.org/10.1093/jscr/rjy351
Close - Share Icon Share
Abstract
Leiomyoma of the anal canal originating from the internal anal sphincter is an extremely rare clinical entity. Generally, it does not produce any clinical signs unless it is large enough to cause obstruction, discomfort, bleeding or pain. The diagnosis is often made incidentally during rectal examination due to other perianal disease or check-up. Herein we report a case of internal anal sphincter leiomyoma diagnosed unexpectedly during rectal examination in a patient with perianal fistula, and treated successfully with surgical excision. We present a review of the literature, the diagnostic strategies, differential diagnosis, prognosis and treatment modalities of this lesion.
INTRODUCTION
Historically, gastrointestinal stromal tumors (GISTs) were incorrectly classified as leiomyomas or leiomyosarcomas [1] because they histologically possessed smooth muscle like structures. With the dramatic advances made in the field of immunohistochemistry, this group of neoplasia were demonstrated to originate from a pluripotential mesenchymal stem cell programmed to differentiate into the interstitial cell of Cajal [2], since then gastrointestinal (GI) mesenchymal neoplasia are classified into two groups, a predominant one which is composed of a collective class of tumors referred to as GISTs and a minor group of true mesenchymal gastrointestinal tract neoplasia, which include lipomas, liposarcomas, leiomyomas, leiomyosarcomas, desmoid tumors, schwannomas and peripheral nerve sheath tumors [3].
CASE REPORT
A 51-year-old male patient with a history of perianal purulent discharge. Physical examination disclosed an external orifice of the perianal fistula located 2 cm far from the anal verge and in the right posterior quadrant. An ovoid mass of ~3 cm in diameter and located 1 cm proximal to the anal verge was palpable at midline posteriorly. The mass was well circumscribed and rubbery in consistency, slightly mobile, in close contact to the anal sphincter.
The MRI scan revealed a well-circumscribed homogeneous mass ovoid in shape, measuring 3.4 × 3.5 × 2.7 cm3 in diameter located in the intersphincteric plane, originating from the posterior aspect of the internal anal sphincter just in the midline, growing away from the lumen, displacing and stretching the external anal sphincter (Figs 1 and 2).
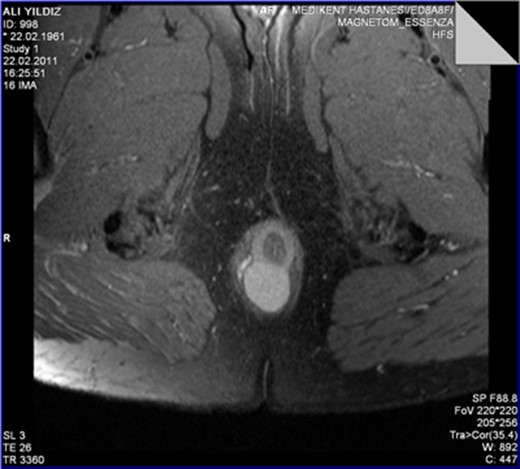
The leiomyoma originating from the internal anal sphincter growing far from the lumen stretching and pushing away the external anal sphincter.
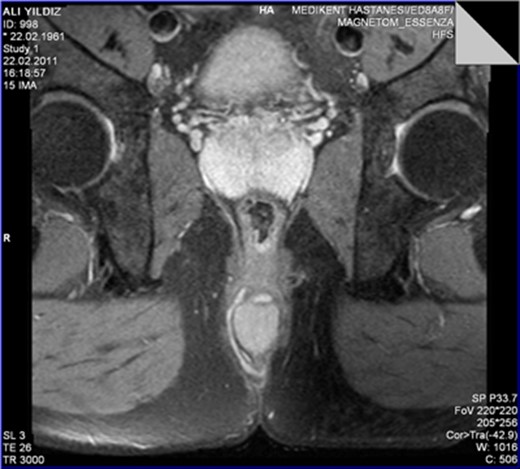
The perinal fistula passing intersphincterically upwards in close relation to the leiomyoma without invasion.
T2A weighed MR sequences showed moderate to low signal intensity, and in post-contrast series there was no internal contrast uptake, necrosis, lymphadenopathy or cystic component. The perianal fistula was intersphincter, fistula tractus passing cranially and very close to the right lateral border of the mass without showing any sign of invasion. During surgery internal orifice of the fistula was identified at the level of dentate line. A stylet was passed through the fistula tractus which was confirmed to be intersphincteric and to be directed cranially in close relation to the right lateral border of the lesion without any signs of invasion. Fistulotomy is performed, dissection was performed meticulously without interruption of the pseudocapsule of the mass, and commenced up to the level where the mass was originating from internal anal sphincter. A few muscle fibers were carefully included into the specimen in order to preserve the integrity of the pseudocapsule and leave the anal sphincter intact. Histopathological examination showed fascicles of uniform spindle cells, with abundant eosinophilic cytoplasm, lack of nuclear atypia, lack of necrosis and practically inexistent mitotic activity (<1 mitoses/50 high power fields). The immunohistochemistry analysis showed a strong positivity for desmin and alpha smooth muscle actin and was negative for S100, CD117 and CD34 stains. This pattern is compatible with the diagnosis of leiomyoma. Postoperative course was uneventful. No complaints of anal incontinence were described by the patient. Patient is now symptom free for 8 years of follow-up.
DISCUSSION
The most common sites for leiomyomas in the gastrointestinal tract are the esophagus and the rectum. In the esophagus, leiomyomas usually appear as an intramural mass located at the distal one-third where there is smooth muscle, or as a small polypoid lesion arising from the muscularis mucosa in the rectum [4]. Most leiomyomas of the large bowel and rectum grow endoluminally whereas tumors of the anal canal tend to grow away from the lumen [5]. Symptoms range from gastrointestinal bleeding, to a palpable mass or anorectal pain [4]. The perianal region is a very rare site and proximity to the sphincter complex can have considerable implications for operative management [6].
This silently growing tumor is usually diagnosed incidentally, during routine physical examination or colonoscopy, or presents with other complaints attributable to perianal region such as hemorrhoids. But to our knowledge, our patient is the first case of true perianal leiomyoma presented as perianal fistula. The differential diagnosis of leiomyoma is ambiguous and comprises a number of different mesenchymal tumors such as schwannomas, leiomyosarcomas and GISTs. For diagnosis work up reasonable demarche is mandatory which usually begins with a careful physical examination and radiological exams such as MRI or high-resolution endorectal ultrasonography. Proctoscopy may be helpful to eliminate other pathologies. Surgical technique must ensure resection with careful dissection to prevent any damage to the anal sphincter complex and to preserve the integrity of the pseudocapsule in order to prevent recurrence. Unlike GISTs, deep soft tissue leiomyomas have a low recurrence rate if local excision is complete [7]. Immunohistochemical analysis is truly an essential step in the definite diagnosis of these lesions and need to be performed on all mesenchymal tumors, because prognosis and therapy may differ greatly depending on the positivity of the immunomarkers and biological behavior of the tumor. A true leiomyoma which is actin and desmin positive with negative c-KIT reaction and negative S100 requires no further treatment other than excision. On the other hand, in the case of even an early stage GIST which stains negative for desmin and actin and positive for c-KIT and CD34, strict follow up is required. In such cases of GISTs with aggressive nature—tumor size ( >5 cm), mitotic activity (<5/50 HPF), tumor margin rupture—further medical treatment with tyrosine kinase blockers such as imatinib mesilate, and even surgical reexcision with a wider margin may be required [8].
Research of late studies about perianal true leiomyomas originating from the anal sphincter is in the order of few cases. This silently growing tumor is usually diagnosed incidentally, during routine physical examination, colonoscopy or other complains of this region such as hemorrhoids, but as a fistula it has never been published yet, in our case the main complain was a purulent intermittent malodorous perianal discharge due to a long time existing productive perianal fistula. The cumulative experience of many authors of this subject recognized that all GISTs have some malignant potential which led them to categorize this group as either low-, intermediate- or high-risk based upon tumor size and mitotic count [9].
CONCLUSION
Any solid mass located in the perianal region is assumed to be potentially malignant even in the presence of benign radiological findings unless otherwise proved immunohistochemically. Owing to the overlapping clinical and gross pathological features between leiomyomas and early stage GISTs the differential diagnosis is extremely difficult so correlated morphologic and molecular studies are necessary to determine and predict the biological behavior of this neoplasia and thus planning further treatment if any is required.
CONFLICT OF INTEREST STATEMENT
None declared.



