-
PDF
- Split View
-
Views
-
Cite
Cite
Atsushi Fushimi, Satoki Kinoshita, Rei Kudo, Hiroshi Takeyama, Incidental discovery of follicular lymphoma by sentinel lymph node biopsy and skin-sparing mastectomy for Paget’s disease associated with invasive breast cancer, Journal of Surgical Case Reports, Volume 2019, Issue 1, January 2019, rjz008, https://doi.org/10.1093/jscr/rjz008
Close - Share Icon Share
Abstract
In breast cancer surgery, establishing a diagnosis other than lymph node metastasis of breast cancer, when performing a sentinel node biopsy in individuals with breast cancer, is rare. Here, we report a case of incidental discovery of follicular lymphoma by sentinel lymph node biopsy for Paget’s disease associated with invasive breast cancer. A 60-year-old female initially presented with erosion on her left nipple and was clinically diagnosed with stage IA human epidermal growth factor receptor type 2 (HER2)-positive breast cancer and Paget’s disease. Accordingly, skin-sparing mastectomy, sentinel lymph node biopsy, and immediate breast reconstruction were performed. Although an intraoperative pathological examination of frozen sections of lymph nodes presented no evidence of metastasis, it revealed large follicles. Based on immunohistochemistry of the additional lymph nodes, she was diagnosed with follicular lymphoma. Therefore, we initiated chemotherapy for follicular lymphoma followed by trastuzumab. At present, 6 years after the operation for breast cancer, the patient is doing well.
INTRODUCTION
Sentinel lymph node biopsy (SLNB) is a surgical procedure in which the lymph nodes of ‘Sentinels’ are identified and examined for metastasis; Cabanas first reported this approach in 1977 for the treatment of penile cancer [1]. Giuliano et al.[2] reported the application of SLNB in breast cancer surgery in 1944; SLNB is now globally used as evidence for omission of axillary lymph node dissection in clinically node-negative patients with breast cancer.
Herein, we report a case of incidental discovery of follicular lymphoma by SLNB for Paget’s disease associated with invasive breast cancer.
CASE HISTORY
A 60-year-old female with unremarkable medical history presented to her former doctor with a 2-month history of erosion on her left nipple in October 2011. While diagnostic mammography detected a mass without spiculation, ultrasonography of the breast revealed an 8-mm hypoechoic area. Physical examination revealed a 1-cm lump in her left lateral breast and a radially spreading erosion on her left nipple suspected to be Paget’s disease. Touch smear cytology of her left nipple revealed the presence of Paget’s cells. These findings were suggestive of ‘pagetoid carcinoma’ [3, 4]. In addition, thoracic and abdominal computed tomography revealed no evidence of metastases for distant organs and no significant enlargement of the lymph nodes, including axilla. In December 2012, she visited our hospital for immediate breast reconstruction. After 1 month, skin-sparing mastectomy, SLNB, and immediate breast reconstruction using a latissimus dorsi musculocutaneous flap were performed. Using a radioactive substance and blue dye, five sentinel lymph nodes were identified. Although the intraoperative frozen sections of the lymph nodes revealed no evidence of metastasis, large follicles were detected (Fig. 1). Additionally, two more clinically normal lymph nodes were sampled. The tumor cells expressed CD20, CD10, and Bcl-2 (Fig. 2). The patient was diagnosed with follicular lymphoma of morphological grade 1 based on World Health Organization criteria. Conversely, an examination of the breast specimen revealed scirrhous carcinoma (8 × 7 mm; grade 3 of 3; Ki-67, 25%) in the left outer breast and Paget’s disease (pure-type; 13 mm; Ki-67, 30%) in the left nipple. Because there was no continuity between invasive ductal carcinoma and Paget’s disease, a diagnosis of synchronous multicentric breast cancer was made (Fig. 3). No lymphatic or vascular invasion was detected. Tumor cells were negative for estrogen and progesterone receptors, and there was intense overexpression of human epidermal growth factor receptor type 2 (HER2), scored as 3+.
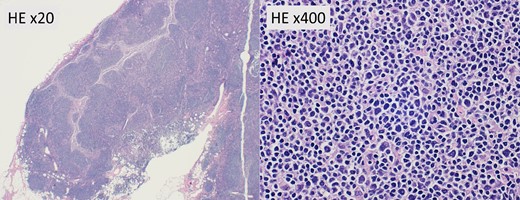
Hematoxylin and eosin staining (HE) of intraoperative frozen sections of lymph nodes showing large follicles without evidence of metastasis. Magnification, ×20, ×400.
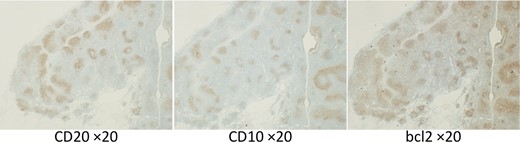
Immunohistochemistry of sampled lymph nodes showing positivity for CD20, CD10, and Bcl-2 protein. Magnification, ×20.
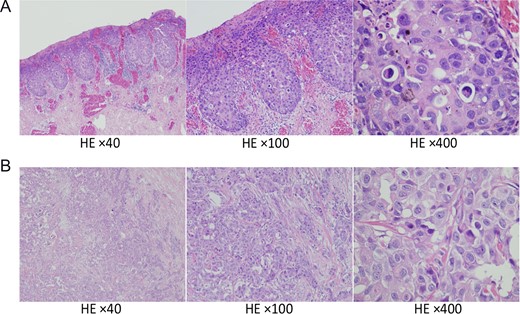
Hematoxylin and eosin staining of surgical specimens. (A) Specimens of the nipple showing Paget’s disease and (B) Specimens of the tumor in the left lateral breast showing scirrhous carcinoma. Magnification, ×40, ×100, ×400.
Additional imaging study performed at the Department of Radiology of our hospital revealed slight enlargement of bilateral supraclavicular and cervical, contralateral axillary, abdominal para-aortic, and inguinal lymph nodes, with a maximum diameter of 22 mm. Thus, the patient was diagnosed with stage III follicular lymphoma.
One month postoperatively, she received eight courses of R-CHOP for follicular lymphoma and attained complete remission 6 months later. Thereafter, she received trastuzumab, for HER2-positive breast cancer, for a duration of one year. At present, 6 years after breast cancer surgery, she is doing well without any recurrence.
DISCUSSION
Paget’s disease of the breast was first reported in 15 cases by Sir James Paget, who demonstrated the association between chronic eczema-like inflammation of the papilla and breast cancer that occurs within 2 years [5]. It is relatively rare for the lesion to be confined only in the papillary area and in the main duct near the papilla, and invasive cancer often coexists in the breast [4]. During surgery for Paget’s disease of the breast, nipple-areolar preservation cannot be achieved. Breast conserving surgery removing the nipple-areola complex is a possibility as long as no other suspicious areas of breast cancer are detected on imaging. In Paget’s disease of the breast, as in standard breast cancer surgery, SLNB has a high identification rate in clinically node-negative individuals [6]. The present study reports a rare case of a patient in whom malignant lymphoma was accidentally detected during SNLB for Paget’s disease associated with invasive breast cancer.
To the best of our knowledge, no study has reported a coexistence between Paget’s disease and malignant lymphoma, and the fact in itself is rare that malignant lymphoma is simultaneously detected during breast cancer surgery. In the literature, there are eight cases of malignant lymphoma diagnosed via the examination of the ipsilateral axillary lymph node during breast cancer surgery (Table 1). In preoperative diagnostic imaging, axillary lymph node enlargement was observed in five of eight patients. Axillary lymph node dissection or lymph node sampling performed during breast cancer surgery led to the diagnosis of malignant lymphoma. The remaining three patients underwent SLNB without clinically lymph node enlargement. Two of three patients were diagnosed with malignant lymphoma from the pathological evaluation of sentinel lymph nodes. The remaining case was node-positive by SLNB; therefore, an additional axillary lymph node dissection revealed follicular lymphoma of the non-sentinel lymph nodes [7].
Cases of malignant lymphoma diagnosed via pathological examination of the ipsilateral axillary lymph node during breast cancer surgery
| Age . | Sex . | Stage of breast cancer . | Surgery . | Histology . | ER . | PgR . | HER2 . | Findings suggestive of lymphoma . | Lymphoma subtype . | Stage of lymphoma . | Therapy for lymphoma . | Year . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 67 | F | pT1bN0M0 | Bp+Sampling | IDC | + | Axillary LN enlargement | MCL | I | None | 2003 | Dutta | ||
| 74 | F | pT2N1M0 | Bt+Ax | IDC | + | + | Ipsilateral axillary LN enlargement | SLL | 0 | None | 2010 | Cuff | |
| 79 | F | pT1aN0M0 | Bt+Ax | Ipsilateral axillary LN enlargement | HL | IA | RT | 2010 | Cuff | ||||
| 54 | F | pT1cN1M0 | Bp+Ax | IDC | + | + | − | Ipsilateral axillary LN enlargement | SLL | 2010 | Cuff | ||
| 49 | F | pTisN0M0 | Bp+Sampling | DCIS | + | + | 2+ | Axillary and para-aortic LN enlargement | FL | III | R-CVP | 2014 | Tamaoki |
| 61 | F | pT1cN0M0 | Bp+SNLB→Ax | IDC | Final pathological examination of non-sentinel LN | FL | III | 2005 | Barranger | ||||
| 74 | F | pT1cN0M0 | Bp+SNLB | ILC | + | + | − | SNLB | FL | I | None | 2010 | Cuff |
| 67 | F | pT1cN0M0 | Bp+SNLB | IDC | − | − | + | SNLB | MCL | IVB | R-DHAP | 2016 | Woo |
| 60 | F | pT1bN0M0 | Bp+SNLB | IDC | − | − | 3+ | SNLB | FL | III | R-CHOP | 2018 | The present case |
| Age . | Sex . | Stage of breast cancer . | Surgery . | Histology . | ER . | PgR . | HER2 . | Findings suggestive of lymphoma . | Lymphoma subtype . | Stage of lymphoma . | Therapy for lymphoma . | Year . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 67 | F | pT1bN0M0 | Bp+Sampling | IDC | + | Axillary LN enlargement | MCL | I | None | 2003 | Dutta | ||
| 74 | F | pT2N1M0 | Bt+Ax | IDC | + | + | Ipsilateral axillary LN enlargement | SLL | 0 | None | 2010 | Cuff | |
| 79 | F | pT1aN0M0 | Bt+Ax | Ipsilateral axillary LN enlargement | HL | IA | RT | 2010 | Cuff | ||||
| 54 | F | pT1cN1M0 | Bp+Ax | IDC | + | + | − | Ipsilateral axillary LN enlargement | SLL | 2010 | Cuff | ||
| 49 | F | pTisN0M0 | Bp+Sampling | DCIS | + | + | 2+ | Axillary and para-aortic LN enlargement | FL | III | R-CVP | 2014 | Tamaoki |
| 61 | F | pT1cN0M0 | Bp+SNLB→Ax | IDC | Final pathological examination of non-sentinel LN | FL | III | 2005 | Barranger | ||||
| 74 | F | pT1cN0M0 | Bp+SNLB | ILC | + | + | − | SNLB | FL | I | None | 2010 | Cuff |
| 67 | F | pT1cN0M0 | Bp+SNLB | IDC | − | − | + | SNLB | MCL | IVB | R-DHAP | 2016 | Woo |
| 60 | F | pT1bN0M0 | Bp+SNLB | IDC | − | − | 3+ | SNLB | FL | III | R-CHOP | 2018 | The present case |
LN: lymph node; IDC: invasive ductal carcinoma; DCIS: ductal carcinoma in situ; ILC: invasive lobular carcinoma; MCL: mantle cell lymphoma; FL: follicular lymphoma; SLL: small lymphocytic B cell lymphoma; HL: Hodgkin lymphoma; RT: radiation therapy; R-CVP: rituximab, cyclophosphamide, vincristine, and prednisolone; R-DHAP: rituximab, dexamethasone, high-dose ara-C-cytarabine, and cisplatin.
Cases of malignant lymphoma diagnosed via pathological examination of the ipsilateral axillary lymph node during breast cancer surgery
| Age . | Sex . | Stage of breast cancer . | Surgery . | Histology . | ER . | PgR . | HER2 . | Findings suggestive of lymphoma . | Lymphoma subtype . | Stage of lymphoma . | Therapy for lymphoma . | Year . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 67 | F | pT1bN0M0 | Bp+Sampling | IDC | + | Axillary LN enlargement | MCL | I | None | 2003 | Dutta | ||
| 74 | F | pT2N1M0 | Bt+Ax | IDC | + | + | Ipsilateral axillary LN enlargement | SLL | 0 | None | 2010 | Cuff | |
| 79 | F | pT1aN0M0 | Bt+Ax | Ipsilateral axillary LN enlargement | HL | IA | RT | 2010 | Cuff | ||||
| 54 | F | pT1cN1M0 | Bp+Ax | IDC | + | + | − | Ipsilateral axillary LN enlargement | SLL | 2010 | Cuff | ||
| 49 | F | pTisN0M0 | Bp+Sampling | DCIS | + | + | 2+ | Axillary and para-aortic LN enlargement | FL | III | R-CVP | 2014 | Tamaoki |
| 61 | F | pT1cN0M0 | Bp+SNLB→Ax | IDC | Final pathological examination of non-sentinel LN | FL | III | 2005 | Barranger | ||||
| 74 | F | pT1cN0M0 | Bp+SNLB | ILC | + | + | − | SNLB | FL | I | None | 2010 | Cuff |
| 67 | F | pT1cN0M0 | Bp+SNLB | IDC | − | − | + | SNLB | MCL | IVB | R-DHAP | 2016 | Woo |
| 60 | F | pT1bN0M0 | Bp+SNLB | IDC | − | − | 3+ | SNLB | FL | III | R-CHOP | 2018 | The present case |
| Age . | Sex . | Stage of breast cancer . | Surgery . | Histology . | ER . | PgR . | HER2 . | Findings suggestive of lymphoma . | Lymphoma subtype . | Stage of lymphoma . | Therapy for lymphoma . | Year . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 67 | F | pT1bN0M0 | Bp+Sampling | IDC | + | Axillary LN enlargement | MCL | I | None | 2003 | Dutta | ||
| 74 | F | pT2N1M0 | Bt+Ax | IDC | + | + | Ipsilateral axillary LN enlargement | SLL | 0 | None | 2010 | Cuff | |
| 79 | F | pT1aN0M0 | Bt+Ax | Ipsilateral axillary LN enlargement | HL | IA | RT | 2010 | Cuff | ||||
| 54 | F | pT1cN1M0 | Bp+Ax | IDC | + | + | − | Ipsilateral axillary LN enlargement | SLL | 2010 | Cuff | ||
| 49 | F | pTisN0M0 | Bp+Sampling | DCIS | + | + | 2+ | Axillary and para-aortic LN enlargement | FL | III | R-CVP | 2014 | Tamaoki |
| 61 | F | pT1cN0M0 | Bp+SNLB→Ax | IDC | Final pathological examination of non-sentinel LN | FL | III | 2005 | Barranger | ||||
| 74 | F | pT1cN0M0 | Bp+SNLB | ILC | + | + | − | SNLB | FL | I | None | 2010 | Cuff |
| 67 | F | pT1cN0M0 | Bp+SNLB | IDC | − | − | + | SNLB | MCL | IVB | R-DHAP | 2016 | Woo |
| 60 | F | pT1bN0M0 | Bp+SNLB | IDC | − | − | 3+ | SNLB | FL | III | R-CHOP | 2018 | The present case |
LN: lymph node; IDC: invasive ductal carcinoma; DCIS: ductal carcinoma in situ; ILC: invasive lobular carcinoma; MCL: mantle cell lymphoma; FL: follicular lymphoma; SLL: small lymphocytic B cell lymphoma; HL: Hodgkin lymphoma; RT: radiation therapy; R-CVP: rituximab, cyclophosphamide, vincristine, and prednisolone; R-DHAP: rituximab, dexamethasone, high-dose ara-C-cytarabine, and cisplatin.
In case of coexistence of malignant lymphoma, it is feared that SLNB would indicate false negative results if lymphoma cells caused occlusion of the lymphatic pathway. Benoit et al. reported a false negative case when breast cancer patients with Waldenström’s macroglobulinemia underwent SLNB; the two sentinel lymph nodes were filled with lymphoplasmacytic cells, and one of the non-sentinel lymph nodes was invaded by breast cancer cells [8].
However, Dy et al. reported that sentinel lymph nodes could be identified in all nine patients with breast cancer with malignant lymphoma who underwent SLNB, among which eight cases had a background of lymphoma in the sentinel nodes [9]. Furthermore, axillary lymph node recurrence was not observed at 37 months of follow-up, including the node-negative cases when omitting axillary lymph node dissection. In addition, Arana et al. argue that malignant lymphoma, particularly small lymphocytic B cell lymphoma, does not inhibit lymphatic flow, and the accuracy of SLNB is maintained even in patients with breast cancer with malignant lymphoma [10].
SLNB can potentially increase the chances of revealing unexpected findings while examining a small number of lymph nodes in more detail compared with that before the era of SLNB. As in our case, when intraoperative pathological examination revealed a finding of malignant lymphoma, it is appropriate to perform additional sampling on non-sentinel lymph nodes to conduct a definitive diagnosis of malignant lymphoma and examine whether it truly is a node-negative case.
Conflict of Interest statement
None declared.
References
- osteitis deformans
- trastuzumab
- immunohistochemistry
- chemotherapy regimen
- follicular lymphoma
- frozen sections
- genes, erbb-2
- hair follicle
- intraoperative care
- mammaplasty
- neoplasm metastasis
- nipples
- ovarian follicle
- epidermal growth factor receptors
- receptor, erbb-2
- sentinel lymph node biopsy
- diagnosis
- lymph nodes
- breast cancer
- lymph node metastasis
- invasive breast cancer
- mastectomy, skin-sparing total
- operative management of breast cancer
- erosion



