-
PDF
- Split View
-
Views
-
Cite
Cite
Rui Marinho, António Alves, Nuno Pignatelli, Vítor Nunes, Unclassified autoimmune pancreatitis mimicking pancreatic cancer, Journal of Surgical Case Reports, Volume 2019, Issue 1, January 2019, rjy340, https://doi.org/10.1093/jscr/rjy340
Close - Share Icon Share
Abstract
A 24-year-old black male presented with a 1-week obstructive jaundice and intermittent abdominal pain, with no significant weight loss and an unsuspicious abdominal exam. Blood chemistry showed a cholestatic pattern but a complete immunological and tumoral panel (anti-smooth muscle antibody, anti-mitochondrial antibody, anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, anti-Smith, anti-double-stranded-DNA antibody (anti-dsDNA), complement C3/C4, carcinoembryonic antigen, CA 19-9 and IgG4) were all within normal limits. Abdominal ultrasound revealed dilatation of the intra and extra-hepatic bile ducts. CT scan showed an abnormal dilatation of the distal bile duct but no focal enlargement of the head of the pancreas. Endoscopic ultrasound suggested an inflammatory process but the magnetic resonance cholangio-pancreatography favored a neoplastic obstruction of the distal common bile duct. Fine-needle aspiration cytology was insufficient for definitive diagnosis and the patient underwent major surgery. Follow-up with mild exocrine pancreatic insufficiency treated with enzyme replacement.
INTRODUCTION
The diagnosis and therapeutic management of autoimmune pancreatitis (AIP) have always been challenging as AIP is a rare pancreatic disorder with a clinical presentation that can sometimes mimic other forms of pancreatitis or even pancreatic cancer [1].
CASE REPORT
A 24-year-old black male presented to the Emergency Department with a 1-week obstructive jaundice and several episodes of intermittent abdominal pain with irradiation to the left upper quadrant and back. The patient denied known gallstones, night sweats, fevers, fatigue, or weight loss and had an unremarkable past medical history.
The abdominal exam revealed a soft, non-distended, non-tender abdomen, without any palpable masses, organomegalies or lymphadenopaties. Blood chemistry showed a cholestatic pattern: bilirubin 6.61 mg/dl, alkaline phosphatase 434 U/l, gamma-glutamyl transpeptidase 374 U/l, alanine transaminase 542 U/l and aspartate transaminase 228 U/l. Lipase, amylase and complete blood count showed regular values. Abdominal ultrasound revealed dilatation of the intra and extrahepatic bile ducts and the pancreas was enlarged and hypoechoic, compatible with inflammation. CT scan was performed to discard a neoplastic obstruction and confirmed an intrahepatic biliary ductal dilatation (Fig. 1) and a dilatation of the distal bile duct with no luminal lesions (Fig. 2).
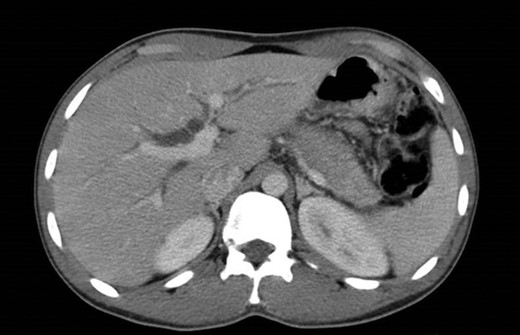
Pancreatic CT scan. General dilatation of the intrahepatic biliary tree and terminal common bile duct.
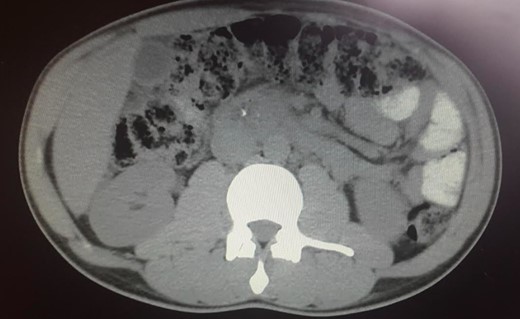
Pancreatic CT scan. Dilatation of the distal bile duct, with no evidence of luminal lesions nor a pancreatic mass constricting the bile duct (endoluminal prosthesis).
Endoscopic retrograde cholangio-pancreatography (ERCP) showed a lobular pancreas with an enhancing heterogeneous pseudonodular mass located in the pancreatic head. The main pancreatic duct (MPD) appeared a well-defined non-beaded narrowing duct. The common bile duct (CBD) showed no narrowing or strictures until the intrapancreatic portion which presented an irregular stenosis of 2 cm length and dilatation upstream the area of stricture.
Laboratory tests including IgG4, anti-smooth muscle antibody (ASMA), anti-mitochondrial antibody (AMA), anti-nuclear antibody (ANA), anti-neutrophil cytoplasmic antibody (ANCA), anti-Sjogren’s-syndrome-related antigen A/B, anti-Smith (anti-Sm), anti-dsDNA, complement C3/C4, CEA and CA 19-9 were all negative. Endoscopic ultrasound (EUS) revealed a heterogeneous parenchyma of the pancreatic head and an EUS-FNA was performed. The MRCP, on the other hand, suggested the existence of a distal cholangiocarcinoma, because of the sudden typical stenosis in the distal CBD with a general dilatation of the upper bile ducts (Fig. 3). Unfortunately, the biopsy was not enough to establish a definitive diagnosis.
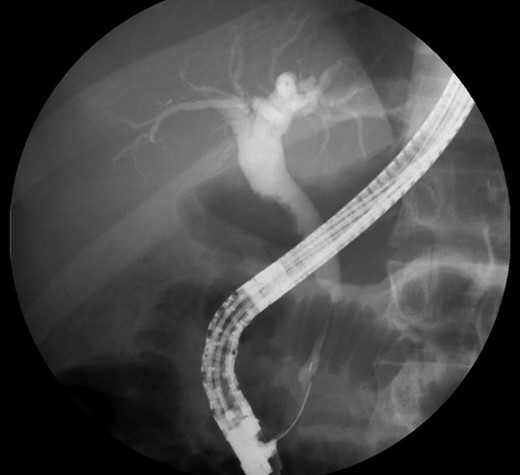
ERCP. Heterogeneous pancreatic parenchyma and pseudonodular appearance of the pancreatic head. Wirsung duct with no stenosis or obstructions. Common bile duct with normal caliber until the intrapancreatic portion where a highly suspicious of malignancy 2 cm irregular stenosis is seen.
The patient was submitted to a pancreaticoduodenectomy. Pathologic intraoperative examination was inconclusive for malignant cells. The pathologic examination suggested a chronic pancreatitis compatible with IgG4-related disease.
DISCUSSION
AIP is a type of rare chronic pancreatitis with a very low prevalence (0,9/ 100,000 individuals) and is twice more frequent in men [1]. The clinical presentation is normally a painless obstructive jaundice, like a pancreatic cancer, and acute pancreatitis is a rare initial presentation.
There are two histologic subgroups of AIP: type 1 or Lymphoplasmacytic Sclerotic Pancreatitis (LPSP) includes dense lymphoplasmacytic infiltrates, organized in a steriform pattern, obliterative phlebitis and mild-to-moderate eosinophil infiltrate. The etiology is not clear, but steroid therapy leads to a permanent relief, so it is crucial to differentiate between AIP and PC. AIP-type 1 is the pancreatic manifestation in the IgG4-related disease spectrum (IgG4RD).
AIP-type 2 is not associated with IgG4RD and shows a classic histological pattern of idiopathic duct-centric pancreatitis (IDCP) with granulocytic epithelial lesions (GEL) [2]. There are no diagnostic tests to diagnose the AIP-type and both are characterized by focal or diffuse pancreatic enlargement accompanied with a narrowing of the main pancreatic duct and IgG4 serum levels cannot establish a definitive diagnosis. The International Consensus Diagnostic Criteria (ICDC) were developed in 2010, assembling the previous criteria [3–7].
Specific features in CT imaging for AIP suspicion are focal or diffuse pancreatic enlargement with loss of the lobular shape (sausage-shaped pancreas), a low-density rim surrounding the pancreas and delayed homogenous enhancement during venous phase [8], which were not present. The patient presented an intrahepatic and distal bile duct dilatation but no pancreatic masses or abnormal enlargements and no delayed homogeneous enhancement. If non-classic CT abdominal features, ERCP can be very helpful in identifying the presence of a narrow stricture (>one-third of the MPD) or multiple non-contiguous strictures and absence of upstream dilatation from the stricture [9]. ERCP showed a pseudonodular mass in the head of the pancreas but the pancreatic duct presented no strictures or irregular stenosis. Only the intrapancreatic portion of the common bile duct presented an irregular 2 cm length stenosis and an upstream dilatation, pointing to cholangiocarcinoma.
Corticosteroid treatments should not be initiated until the possibility of malignancy is excluded, because some subtypes of PC and pancreatic lymphoma can respond to this therapy. CT scan features such as diffusely enlarged sausage-shaped pancreas without a clear ductal pancreatic dilatation render AIP more likely than PC, but if typical neoplastic features are present (e.g. intrapancreatic portion of the common bile duct with an irregular 2 cm length stenosis), patients should be considered as having a PC or cholangiocarcinoma [8]. The sudden stenosis of the distal common bile duct with a general upstream dilatation revealed in the MRCP and ERCP and also the absence of a long (>one-third length of the MPD) or multiple strictures of the MPD were major features for not considering de autoimmune etiology. Routine workup diagnosis for cancer was not negative and the patient undergone pancreatic resection.
Two possibilities can be considered: one is that the patient had really type 1 AIP but in a burn-out stage and another is that the patient’s true diagnosis is an unclassified variant of AIP (mixed type 1 and 2 AIP). Not all cases of AIP fit clearly into the two subtypes. Some cases show the typical triumvirate histologic type 1 disease (i.e. dense lymphoplasmacytic inflammation, storiform-type fibrosis (Fig. 4), and obliterative phlebitis), but they also show more than an occasional intraductal aggregate of neutrophils. Others show typical type 2 disease pattern but with diffuse infiltrates of IgG4+ plasma cells, more than 50/high power filed (HPF) like in our case (Fig. 5). Dense, diffuse infiltrates of IgG4+ plasma cells that number >50/HPF are reportedly highly specific for IgG4RD [10], but definitive diagnosis of type 1 AIP should be based on the triumvirate histology. The absence of IgG4 cells does not necessarily imply the diagnosis of type 2, as type 1 also can be seronegative.
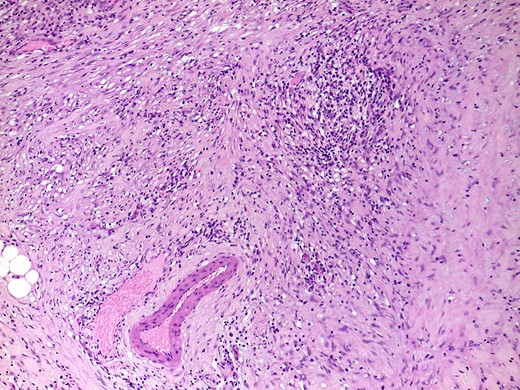
H&E, ×100. Parenchymal fibrosis with focal storiform-type areas and a lymphoplasmacytic infiltrate, that focally involves a vein, without overt obliterative phlebitis.
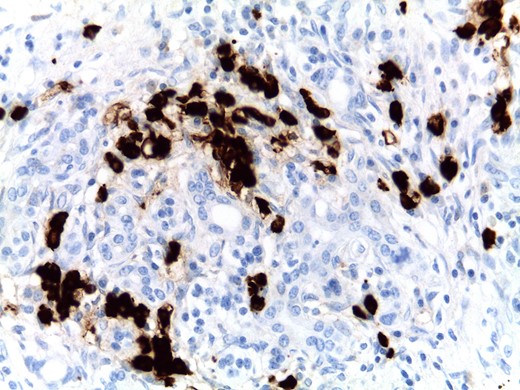
Focal areas with more than 50 IgG4+ plasma cells per high-power field were found, although a diffuse infiltrate was not present in this case.
Patients that underwent pancreatic resection represent a failed or an insufficient routine workup diagnosis (up to 2–6%).
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
REFERENCES
- nuclear magnetic resonance
- abdominal pain
- computed tomography
- antimitochondrial antibody
- weight reduction
- antineutrophil cytoplasmic autoantibody
- antinuclear antibody
- cholestasis
- fine needle aspiration biopsy for cytology
- bile fluid
- bile ducts
- blood chemical analysis
- ca-19-9 antigen
- common bile duct
- complement 3
- dilatation, pathologic
- dna
- follow-up
- surgical procedures, operative
- antibodies
- carcinoembryonic antigen
- diagnosis
- hypertrophy
- pancreas
- surgery specialty
- pancreatic cancer
- jaundice, obstructive
- exocrine pancreatic insufficiency
- abdominal examination
- anti-dsdna antibody
- abdominal ultrasonography
- enzyme replacement therapy
- pancreas head
- pancreatitis, autoimmune
- smooth muscle antibody
- immunoglobulin g4
- endoscopic ultrasound
- pancreatogram
- enzyme replacement or supplement agents



