-
PDF
- Split View
-
Views
-
Cite
Cite
Nimisha Vallabh, Venkat Srinivasan, David Hughes, David Agbamu, Salivary duct carcinoma arising from the inferior turbinate, Journal of Surgical Case Reports, Volume 2017, Issue 7, July 2017, rjx090, https://doi.org/10.1093/jscr/rjx090
Close - Share Icon Share
ABSTRACT
Salivary duct carcinoma (SDC) is an uncommon, aggressive tumour, which carries a poor prognosis. SDC affects the major salivary glands, usually occurring in the parotid gland. A 76-year-old male patient presented with right-sided nasal obstruction and rhinorrhoea. A polypoidal mass was seen in the right inferior turbinate mimicking a benign neoplasm. Histological examination following wide excision led to a diagnosis of SDC. There are no therapeutic guidelines and management is based on experience with SDC of the major salivary glands. Given the aggressive nature and poor prognosis of the disease, it is a rare but important differential to consider in patients with unilateral nasal mass.
INTRODUCTION
Most unilateral nasal masses are benign. Common causes include nasal polyp, inverted papilloma and squamous papilloma [1]. Primary malignant tumours of the nasal cavity are rare comprising 0.5% of all cancers [2]. The most common are squamous cell carcinoma and adenocarcinoma [3]. While sinonasal tumours are a uncommon, it is vital that these patients are identified and referred for prompt specialist assessment. Salivary duct carcinoma (SDC) is a rare, aggressive tumour, which carries a poor prognosis. SDC affects the major salivary glands, usually occurring in the parotid gland.
CASE REPORT
A 76-year-old male patient was referred to the ENT department with a history of right-sided nasal obstruction and rhinorrhoea. Clinical examination, revealed a polypoidal mass in the right inferior turbinate and deviation of nasal septum to the right. He had a past medical history of hypertension, angina and benign prostatic hypertrophy. A computed tomography (CT) scan of the paranasal sinuses confirmed the swelling of the right inferior turbinate, touching the nasal septum and floor, in addition to the septal deflection (Fig. 1). Clinically, a diagnosis of benign nasal tumour, most likely inverted papilloma, was made. As the lesion was not suspicious of a malignant pathology, a pre-operative magnetic resonance imaging (MRI) was not requested. The patient elected to have a wide local excision of the lesion. A septoplasty was carried out to allow access. Intra-operative findings confirmed a large polypoidal mass originating from the right inferior turbinate extending into the adjacent part of the lateral wall of the nose. It was felt that surgical excision with good margins of clearance was achievable; therefore, endoscopic medial maxillectomy including removal of inferior turbinate was performed. The patient recovered well from the procedure and was discharged the following day.
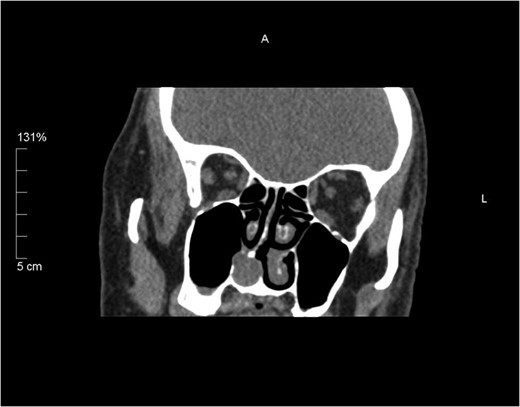
Coronal unenhanced CT sinus showing a 15 mm rounded soft tissue lesion arising from the right inferior turbinate thinning the medial wall of the right maxillary antrum and slightly bulging in to the sinus.
Histology of the mass confirmed sinonasal SDC. Tissue specimens showed cellular stroma extensively infiltrated by carcinoma composed of large eosinophilic cells with gland formation (Fig. 2). Widespread nuclear pleomorphism was evident. The sample was positive for cytokeratin-7 (CK7) (Fig. 3), epithelial membrane antigen (EMA), BerEP4 and demonstrated focal positivity for cytokeratin-5/6 (CK5/6). Post-operative MRI scans were requested to rule out residual disease or cervical lymphadenopathy (Fig. 4). Following review by Regional Head and Neck Cancer multi-disciplinary team and the oncologist, the patient was treated with a radical course of radiotherapy which was completed without complications. The patient has been reviewed regularly in the Head and Neck Cancer clinic with no evidence of recurrence at 18 months.
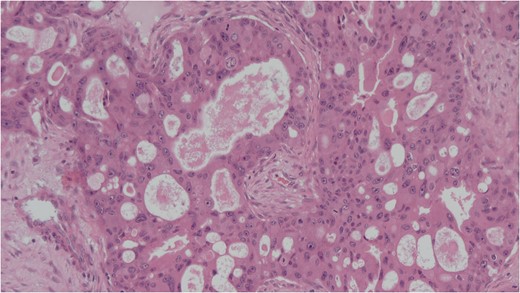
Cellular stroma extensively infiltrated by carcinoma composed of large eosinophilic cells with gland formation on medium power view. Haemotoxylin and eosin (H&E).
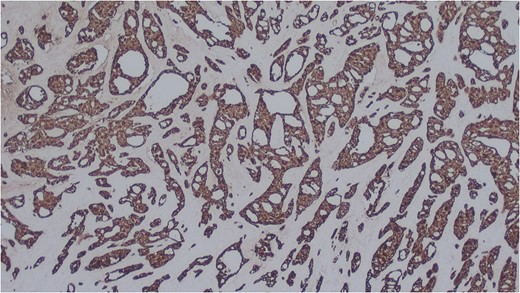
Salivary duct carcinoma cells highlighted by immunohistochemistry. CK7 (x25).
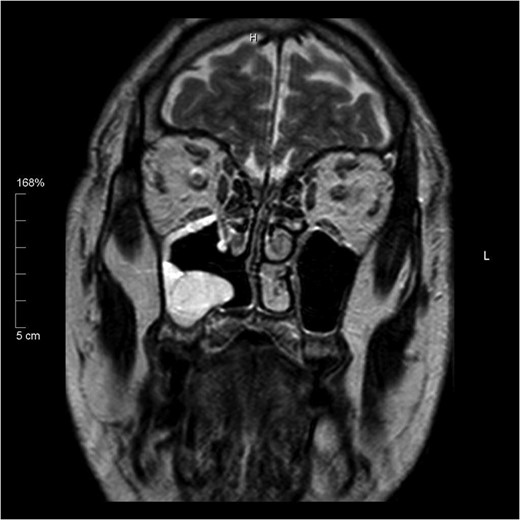
Coronal T2 image showing post-operative changes. No evidence of residual tumour.
DISCUSSION
SDC was first described in 1968 by Kleinsasser et al. [3]. It arises from the major salivary glands and occurs in patients in the fifth or sixth decade, presenting as a rapidly enlarging firm mass. Men are affected three times as often as women [4]. Due to its fast growing nature, over 25% of patients will have facial nerve dysfunction or paralysis at presentation; one-third will have cervical lymphadenopathy [4]. SDC occurs in the parotid gland in 88% of cases, with the submandibular gland only affected in 8% of cases [4, 5]. Other rarer sites include the sublingual gland and minor salivary glands in the palate, retromolar trigone, tongue [4] and larynx [6]. Two cases of SDC of the sinonasal tract have been reported in the literature. The first case was thought to have originated from the paranasal sinuses or lacrimal duct and the patient had multiple pulmonary metastases at presentation [7]. The second reported case presented with nasal obstruction and epistaxis with a lesion seen in the left inferior meatus [8].
Clinically, it may be difficult at times to differentiate SDC from more common tumours of the sinonasal tract. The radiological features of SDC are non-specific; however, pre-operative imaging may help to diagnose malignancy. The histological appearance of SDC resembles ductal breast carcinoma. Macroscopically, SDC is poorly circumscribed, unencapsulated and usually multinodular. Microscopically, intraductal and infiltrating components can be seen which resemble ductal breast carcinoma [4]. Comedo necrosis and calcifications are characteristic of SDC [4]. In contrast to ductal breast carcinoma, the majority of SDC are androgen receptor positive and oestrogen and progesterone receptor negative [4, 5]. Epithelial membrane antigen, cytokeratin-7 (CK7) and EMA are usually positive in SDC [5]. These features can help to differentiate SDC from the more common squamous cell carcinoma. Squamous cell carcinoma will demonstrate evidence of squamous differentiation and stain positively for cytokeratin-5/6. The 5-year survival rate for squamous cell carcinoma is 69% [9], which is significantly better than SDC. As it is more common, treatment protocols are better established.
SDC carries a high incidence of loco-regional and distant metastases and therefore a poor prognosis [4]. Surgical excision is typically the first stage in the management of this disease. A review by Wee et al. [4] suggested a neck dissection should be carried out in all patients presenting with N0 or Nx disease as SDC metastasises to the neck in 65% of patients. The role of post-operative radiotherapy is difficult to determine given the small numbers of patients [4].
There are several unusual features in the case presented here. Our patient’s primary symptom was unilateral nasal blockage. Clinically, the nasal mass was smooth and polypoidal in appearance mimicking a benign pathology. The differential diagnosis included nasal polyp, inverted papilloma, haemangioma and schwannoma. Traditionally, SDC grows rapidly, resulting in the majority of patients presenting with advanced stage disease. Our patient was diagnosed at an early stage with no symptoms to suggest rapid growth. The histological diagnosis of SDC was unexpected given the above clinical features. Our patient was managed with wide local excision of the tumour followed by post- operative radiotherapy once a histological diagnosis of SDC had been confirmed. As there are so few cases of SDC of the sinonasal tract, there are no therapeutic guidelines and management is based on experience with SDC of the major salivary glands. Given the aggressive nature and poor prognosis of the disease, it is a rare but important differential to consider for a mass in the sinonasal tract.
CONFLICT OF INTEREST STATEMENT
None declared.



