-
PDF
- Split View
-
Views
-
Cite
Cite
Klodian Krakulli, Edvin Prifti, Vinicio Fiorani, Mario Zogno, Successful surgical employment of Impella recovery system for right ventricular failure after previous aortic valve replacement, Journal of Surgical Case Reports, Volume 2017, Issue 5, May 2017, rjx091, https://doi.org/10.1093/jscr/rjx091
Close - Share Icon Share
ABSTRACT
A 58-year-old woman underwent aortic valve replacement. On the second postoperative day the patient referred a sharply chest pain, and an emergent coronary angiography revealed total occlusion of the right coronary artery. An intra-aortic ballon pump was placed and the patient underwent emergent off-pump coronary revascularization of the right coronary artery. Five hours later, due to unstable hemodynamic the extracorporeal membrane oxygenation was implanted without improvement of the right ventricular (RV) function. Then we decided to implant the Impella Right Direct (RD). After 9 days of Impella’s insertion the RV was recovered and the device was successfully explanted. After 16 days of Impella explanted the patient was discharged. This case suggest that implantation of Impella RD is clinically feasible, associated with hemodynamic improvement, and facilitate successful bridge-to-recovery in patients with post-cardiotomy RV failure due to myocardial infarction unresponsive to coronary artery bypass grafting, maximal medical therapy, contrapulsation and extracorporeal membrane oxygenation.
INTRODUCTION
Acute right ventricular (RV) failure is associated with significant morbidity and mortality [1], which may develop as a consequence of acute myocardial infarction, pulmonary embolism, myocarditis, ventricular septal defect or post-cardiotomy [2, 3]. Despite optimal medical management, some patients fail to improve and require implantation of a RV assist device. The RV may exhibit a greater capacity for rapid recovery compared with the left ventricle [3]. Recent studies suggests that most of the patients recover sufficient function to allow explantation of the Impella Recovery Right Direct (IRRD) or Right Peripheral was reported [4]. Herein we report a case of successful implantation of the IRRD in a patient with acute RV failure after previous aortic valve (AV) replacement despite CABG, maximal medical therapy, intra-aortic balloon counterpulsation (IABP) and extracorporeal membrane oxygenation (ECMO).
CASE PRESENTATION
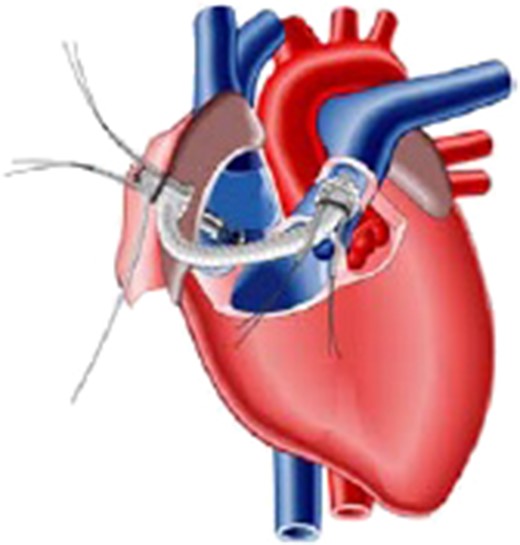
Schematic presentation of the Impella Right Direct implantation.
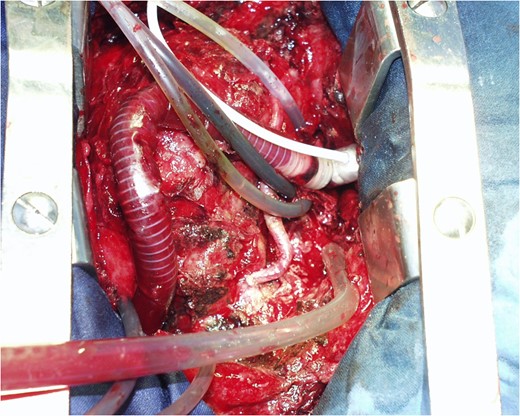
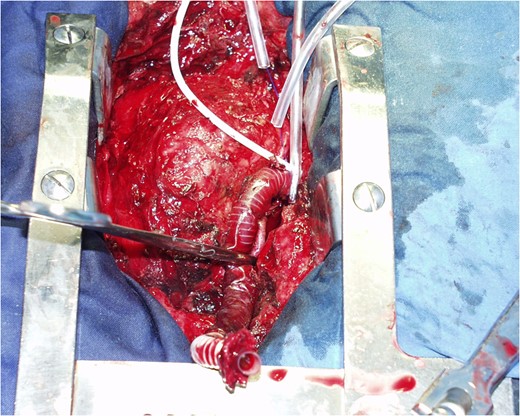
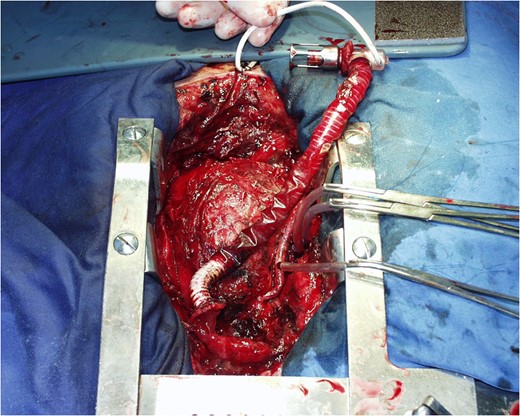
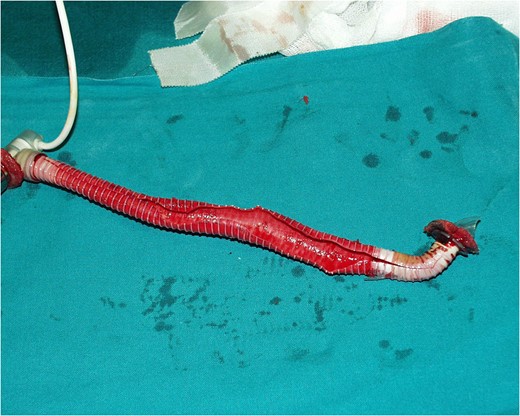
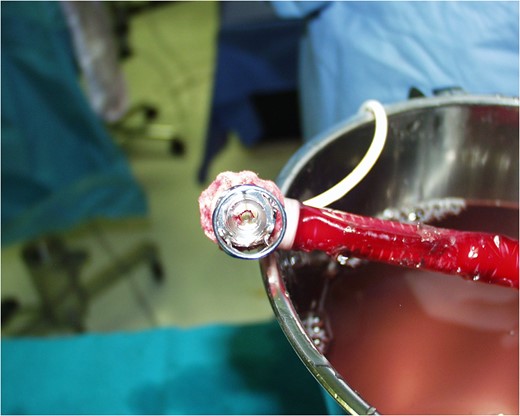

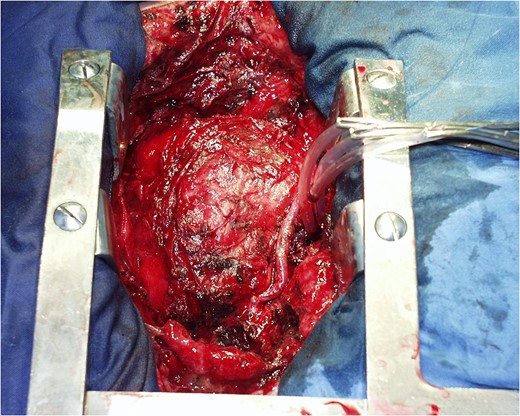
COMMENTS
Acute RV failure is an increasingly common clinical problem and despite optimal medical management, some patients fail to improve and require implantation of a RV assist device, which improves end-organ perfusion and provides an opportunity for reversal of multisystem organ failure. The IRRD is a microaxial pump designed for temporary RV support. Schmidt et al. [5] first described its use in 2003, employing such a device in 8 patients and later Christiansen et al. [6] in other two patients. Subsequently, the device has been used post-cardiotomy, post-transplant, and post left ventricular assist device implantation [7].
We have described the employment of the IRRD in a patient with RV failure due to myocarcial infarction post AV replacement, unresponsive to CABG. There is growing evidence to suggest that the IRRD can be successfully used for the treatment of refractory acute RV failure. The reasons why we choose this device were: (i) easy to insert, (ii) accommodates patients of all size (flow rate up to 5.5 l), (iii) minimal anticoagulation, (iv) minimal hemodilution, (v) minimal destruction of blood or plasma components, (vi) possibility of sternal closure and (vii) reduction of myocardial workload and oxygen consumption.
Important aspects of blood pumps are lifetime, costs, adaptability to diverse applications and patient requirements, rapid and easy deployment, thrombogenicity, flow characteristics and blood damage [8]. The major advantages of the IRRD are its small size, the simple design, the low energy requirements, and the avoidance of a priming volume. These figures lead to a reliable pump function without technical failures and may result in a reduced number of transfusion requirements which is reported to be excessively high in patients with ECMO support [9]. Flow and pressure characteristics, shear stress, blood exposure times to artificial surfaces and the size of the pump are important factors contributing to hemolysis.
The effects of pulsatile and non-pulsatile flow on pulmonary vasculature remains controversial. Some studies demonstrated beneficial effects of pulsatile flow [10], others could not confirm these results [11]. However, the pulsatile flow results in a decreased pulmonary vascular resistance. This might be due to release of endothelium-derived relaxing factor by rhythmic stimulation of endothelial cells through oscillating changes in vessel wall shear stress [10]. This effect of pulsatile flow does not appear to improve pulmonary gas exchange, peak inspiratory pressure, mean PA pressure, oxygenation capacity and development of pulmonary edema [11]. It seems to be justified to support the RV with non-pulsatile flow devices, especially when support duration is limited to a few days as in patients with post-cardiotomy failure.
The main disadvantage of the IRRD is that implantation requires a sternotomy and direct cannulation of the right atrium and PA. This increases the risk of bleeding and infection and necessitates an additional operation for device explantation. These risks are not insignificant, considering that patients requiring implantation of an RV assist device are frequently critically ill with multisystem organ failure and may be coagulopathic due to hepatic congestion and/or the presence of anti-platelet medications [7]. The Impella Right Peripheral is an alternative to IRRD which allows the percutaneous implantation of the device. The Margey et al. [12] used this device for temporary RV mechanical circulatory support in a similar patient presenting with an inferoposterior ST-segment elevation myocardial infarction, who despite revascularization, optimized medical therapy, IABP, remained in profound cardiogenic shock.
We have described the clinical outcome in our patient with refractory acute RV failure supported with the IRRD. Our findings suggest that implantation of these devices is clinically feasible, associated with hemodynamic improvement, and facilitate successful bridge-to-recovery in most patients with post-cardiotomy RV failure due to myocardial infarction unresponsive to CABG, maximal medical therapy, IABP and ECMO.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest regarding the publication of this article.
REFERENCES
- aorta
- myocardial infarction
- coronary angiography
- coronary artery bypass surgery
- heart failure, right-sided
- hemodynamics
- extracorporeal membrane oxygenation
- chest pain
- coronary revascularization
- right coronary artery
- aortic valve replacement
- employment
- heart ventricle
- surgical procedures, operative
- medical devices
- doppler hemodynamics
- implants
- medical management



