-
PDF
- Split View
-
Views
-
Cite
Cite
Sudeep Khaniya, Vikal Chandra Shakya, Rabin Koirala, Solid pseudopapillary tumor in an ectopic pancreas: an unusual presentation, Journal of Surgical Case Reports, Volume 2017, Issue 3, March 2017, rjx050, https://doi.org/10.1093/jscr/rjx050
Close - Share Icon Share
Abstract
Pancreatic solid pseudopapillary tumor is a rare neoplasm. Very rarely, it may arise from an ectopic site. Solid pseudopapillary tumor occurring in the root of mesentery has not been described in the literature. This report summarizes a case of an adult male having the tumor arising from the mesenteric root. He underwent complete resection of the tumor followed by six cycles of adjuvant chemotherapy and remains asymptomatic till date.
INTRODUCTION
Pancreatic solid pseudopapillary tumor (SPT) is a rare neoplasm, representing ~2% of pancreatic tumors. The histological origin of the tumor is poorly understood, showing remarkable variability in their presentation, biological behavior and prognosis [1]. The occurrence of SPT at an ectopic pancreatic tissue is even more unusual. Our literature search has identified only 16 previously reported cases of SPT at an ectopic site [2–5]. We report a case of 33-year-old male presenting with SPT arising in the root of small bowel mesentery with lymph-node metastasis. The site as well as the presence of lymph-node metastasis makes our case report unusual.
CASE REPORT
A 33-year male presented with a 1-year history of epigastric and right upper abdominal pain associated with episodes of upper abdominal fullness relieved after vomiting. He had history of 4 kg weight loss over a period of 3 months. Examination revealed a mass of size 5 × 7 cm over the epigastric region.
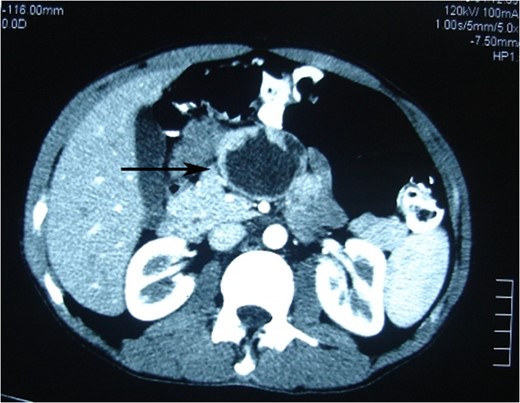
Photomicrograph of the arterial phase CT showing well-circumscribed low attenuated lesion (arrow).
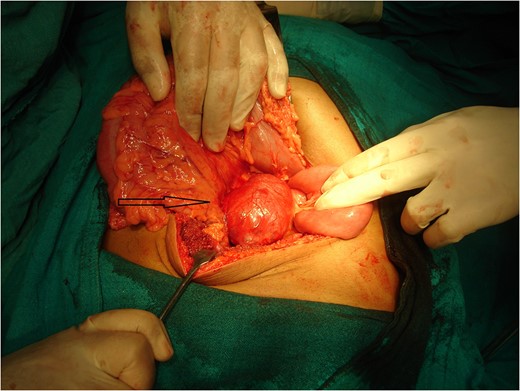
A photograph during laparotomy showing the origin of the lesion (arrow) at the root of mesentery medial to the duodenojenunal flexure.
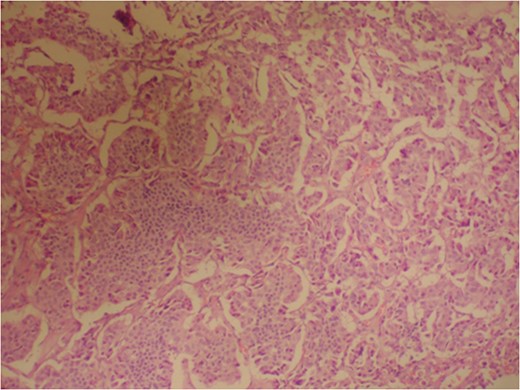
Photomicrograph indicating the tumor composed of irregular pseudopapillary structures; prominent myxoid changes can be seen in the stroma. (hematoxylin and eosin stain, ×200).
The patient had uneventful postoperative period and was discharged on eighth postoperative day. He received six cycles of gemcitabine (1 g/m2). Till the end of 10 months, he has remained asymptomatic.
DISCUSSION
SPTs of the pancreas are rare exocrine pancreatic tumor, well known for indolent biologic behavior. Frequently, it is named as solid and cystic tumor, solid and papillary epithelial neoplasm, papillary-cystic neoplasm, papillary-cystic epithelial neoplasm, papillary-cystic tumor or Franz tumor [6]. In 1996, the World Health Organization renamed this tumor as SPT for the international histological classification of tumors of the exocrine pancreas. It commonly occurs in the head or tail region of the pancreas, usually in females in second to fourth decade of life [7].
The present case was a 33-year-old male who had presented with an epigastric mass. Radiological investigations showed a cystic lesion with irregular walls below the body of pancreas. Laparotomy revealed the lesion to be arising from the root of small bowel mesentery medial to the duodenojejunal flexure, with a nearby lymph node; the lesion being separate from the normal pancreas. Histopathological examination showed that the lesion was an SPT of pancreatic origin and had also metastasized to the lymph node. SPT occurring in an ectopic pancreas is a rare phenomenon, with only 16 previously reported cases, at various sites such as the mesocolon, ovary, retroperitoneum, liver, stomach and duodenum [2–5]. The root of small bowel mesentery is till date an unreported site for SPT; this being the first such case described. The presence of tumor in male and the lymph-node metastasis is also a rare occurrence. The clinical presentation of the tumor is usually nonspecific, like vague abdominal pain, gradually enlarging mass or compressive signs [7]. Some asymptomatic patients are detected incidentally during routine physical examination or by imaging studies. In some rare instances, acute manifestations such as pancreatitis or hemoperitoneum caused by rupture of the tumor has been the presenting symptom [8, 9].
Ultrasonographically, the tumor is well encapsulated, homogeneous or heterogeneous, and composed of solid echogenic and hypoechogenic cystic components [10]. CT scan usually demonstrates a well-encapsulated and circumscribed retroperitoneal mass, hypodense, with various solid and cystic components owing to hemorrhagic degeneration [11]. Magnetic resonance imaging is better than CT for distinguishing certain tissue characteristics, such as hemorrhage, cystic degeneration or the presence of a capsule. Fine needle aspiration cytology has been reserved for selected cases [10].
The pathologic diagnosis of SPT on light microscopy depends upon the presence of solid areas alternating with pseudopapillary formations; evidence of cellular degeneration, cholesterol clefts, nuclear grooves, aggregates of foamy histiocytes and hyaline cytoplasmic globules. Immunohistochemical staining for specific cell lineage markers is of marginal utility in proving the diagnosis of SPT. Vimentin is consistently expressed, and tumor cells are focally positive for cytokeratin and synaptophysin, and few positive for S-100 protein, CD-56 and CD-10 [7, 12].
The management of SPT localized to the pancreas is straightforward and surgical removal with or without pancreatic resection is the treatment of choice. Extensive radical dissection is not indicated when the disease is localized. Similarly, local invasion and metastasis should not be the contraindication for resection [7, 12]. The benefit of chemotherapy or radiotherapy is poorly understood. There have been reports of using various chemotherapy regimens based upon cisplatinum and 5-flururacil, gemcitabine alone or combination of ifosfamide, cisplatin and VP-16 [12]. Adjuvant radiotherapy has been used in some unresectable cases with good results. Usually, these tumors have good prognosis with 5-year survival rate of ~95% [7].
CONCLUSION
SPT remains a distinct entity and has favorable prognosis, though histogenesis is still controversial. The small bowel mesentery can be a site of such ectopic pancreatic tumor.
CONFLICT OF INTEREST STATEMENT
None declared.



