-
PDF
- Split View
-
Views
-
Cite
Cite
Konstantinos Kothonidis, Fadi Maassarani, Yves Couvreur, Bernard Vanhoute, Robert De Keuleneer, Primary anorectal melanoma—a rare entity: case report, Journal of Surgical Case Reports, Volume 2017, Issue 3, March 2017, rjx060, https://doi.org/10.1093/jscr/rjx060
Close - Share Icon Share
Abstract
Anorectal melanoma is a rare disorder. There have only been a few cases reported and there is no consensus of treatment. We report a case of 50-year-old Caucasian man presenting intermittent prolapse of an anorectal mass during 6 months with occasional bleeding. Biopsies came up with the diagnosis of malignant melanoma. No distant metastases were found. He underwent an abdominoperineal resection due to internal sphincter invasion. A second tumour was diagnosed in final histologic examination: a dysplastic rectal polyp invaded by the melanoma (collision tumour). At 12 months of follow up he presented loco-regional recurrence (a single pelvic lymph node) and hepatic metastases. He was included in a study protocol comparing new medical treatments (nivolumab versus ipilimumab or both).
INTRODUCTION
Anorectal malignancies are commonly adenocarcinoma or squamous cell carcinoma, with melanoma having a relative incidence of 0.5–4% of all malignancies in this region [1, 2]. This site is the third most common primary location for melanoma after skin and retina and yet only 0.4–1.6% of all primary melanomas arise here [2, 3].
Primary anorectal melanoma (PARM) is a rare disorder. About 1% of all anorectal carcinomas are melanomas, typically presenting in the fifth or sixth decade of life and predominantly in women.
Patients present themselves with local symptoms like rectal bleeding and a changed defecation pattern.
Prognosis is very poor with a median survival of 24 months and a 5-year survival of 10%. Almost all patients die because of metastases.
Due to rarity of this entity there is no consensus on which surgical approach is favourable. The surgical procedure of choice ranges from an abdominoperineal resection (APR) to wide local excision (WLE) with or without adjuvant radiotherapy.
CASE REPORT
We report a case of PARM in a 50-year-old Caucasian man presenting intermittent prolapse of an anorectal mass during 6 months with occasional bleeding. The patient was in good condition with no weight loss and past medical and family history unremarkable.
Proctoscopy confirmed the physical examination and showed a 3 cm bleeding villous polyp that originated above the dentate line. Endorectal ultrasound showed a fusion of all layers, infiltration of the sphincters and no suspect lymph nodes. Magnetic resonance imaging of the pelvis highlighted a high suspicion of internal sphincter infiltration and no inguinal or pelvic lymph nodes. Positron emission tomography–computed tomography (PET–CT) showed an intensely hypermetabolic lesion centred on the lower rectum (standardized uptake value (SUV) = 35.1) and no evidence of disease extension loco-regionally or at distance (Figs 1 and 2).
The histopathology of the biopsy concluded to a primitive malignant melanoma due to the tumour profile in immunohistochemistry: Human Melanoma Black (HMB-45) +++, S100 protein +++, Melan A +, Wide spectrum cytokeratins negative.
The oncologic multidisciplinary council validate a surgical treatment without neoadjuvant radio or chemotherapy. Due to high suspicion of internal sphincter involvement an APR was performed along with a left-sided end colostomy. The post-operative period was uneventful.
The histopathology reported a pedicle, mostly exophytic tumour of the anorectal junction, measuring 2.5 × 2.5 × 1.5 cm3. This tumour is associated to a tubulo-villous polyp in low grade dysplasia coexisting on the tumour pedicle. The tumour was of mixed histology, associating epithelioid and spindle cells. The superficial muscular layer was invaded. The nine lymph nodes examined and all resection margins were free of tumour. No perineural invasion was revealed. There was no BRAF mutation identified on genetic analysis.
At twelve months of follow up, the patient presented a single hypermetabolic lymphadenopathy localized in the left obturator region, measuring 13 mm long axis (SUV = 9.5) and several spots of increased glycolytic activity in the hepatic parenchyma, which only one was corresponding to a small hypodense lesion (SUV = 4.5). The patient was included in a study protocol comparing a new treatment anti-PD1 (nivolumab) with Ipilimumab (monoclonal antibody anti-CTLA-4) or both.
DISCUSSION
PARM is a rare disorder. About 1% of all anorectal carcinomas are melanomas, typically presenting in the fifth or sixth decade of life and predominantly in women. It may be difficult to identify, and may be misdiagnosed as a haemorrhoid, rectal polyp, or as an ulcerative lesion following prolapse through the anal orifice.
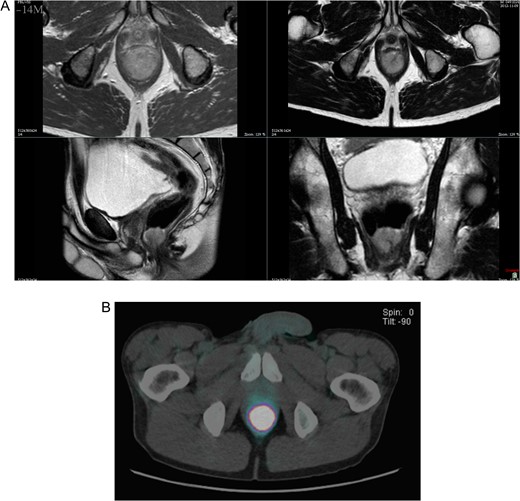
(A) T2-weighted axial, coronal and transverse MRI images, demonstrating the polypoid 3 cm anorectal mass. (B) PET–CT showed an intensely hypermetabolic lesion centered on the lower rectum.
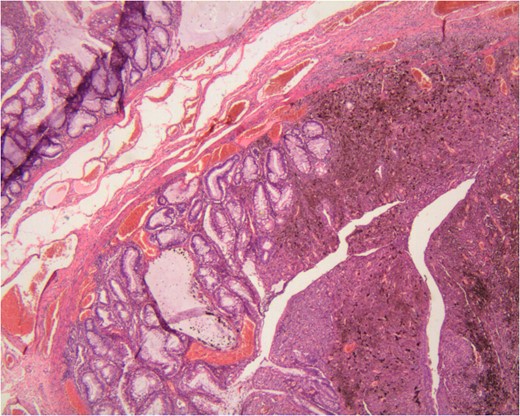
The rarity of this case is based on the observation of a tubulo-vilous polype on the tumour pedicle. Actually there was a small in situ melanoma on the anal side that turn into a polypoid and invasive melanoma upwards to the rectal mucosa. It infiltrated the dysplastic polyp that was on its way. We can therefore call this a ‘collision tumour’. We do not know to this date if there is any association of those two tumours on the anorectal melanoma pathogenesis [6].
To our knowledge, there is no other similar observation reported in the exception of case reporting synchronous anorectal melanoma and rectal adenocarcinoma in a distance of 3-4 cm between them [7].
In the absence of metastasis surgical therapy is the treatment of choice. There is no consensus on which surgical approach is preferred between WLE and APR. However, several recent studies suggest that, if possible, sphincter-sparing local excision and adjuvant radiation is well tolerated and can effectively control loco-regional disease while avoiding the functional morbidity of the APR [3–5, 8, 9]. On the other hand, mesorectal lymph nodes are involved in preference to inguinal lymph nodes in contrast to squamous cell carcinoma of the anus. If an APR is performed, the mesorectal lymph node resection may contribute to a better staging of the disease. There is no value of prophylactic inguinal lymph node resection [2, 3, 5].
Patients without lymph node metastasis have a survival advantage with a 5-year survival rate of 20 versus 0% in patients with metastasis. Survival of patients with reccurent or metastatic disease is <10 months [10]. There is an important deterioration of the prognosis for tumours superior to 20 mm. A worse prognosis was also associated with tumour thickness, tumour necrosis (important histologic feature, representing a biologically more aggressive tumour) and perineural invasion [5]. An amelanotic lesion has a worse prognosis [2]. This reported survival is not up to date due to the small number of cases and does not take into account new medical treatments (nivolumab and Ipilimumab).
CONFLICT OF INTEREST STATEMENT
None declared.



