-
PDF
- Split View
-
Views
-
Cite
Cite
Rocco Severino, Paolo Severino, Reasons why a second radiological opinion is advisable: a case report of a misreported crural synovial cell sarcoma, Journal of Surgical Case Reports, Volume 2017, Issue 3, March 2017, rjx046, https://doi.org/10.1093/jscr/rjx046
Close - Share Icon Share
Abstract
Synovial sarcoma (SS) is a rare condition that accounts for 5–10% of all soft-tissue sarcomas (STS). SS locates most frequently near the joints, in particular at the lower extremities, but it can also occur in other locations. We report a case of a 42-year-old male complaining of a slow-growth mass on his right thigh, reported as a femoral nerve shwannoma on the basis of the preoperative radiological investigations, which revealed to be a monophasic SS on the histological examination. During the surgical procedure, the assistance of vascular surgeons was required to reconstruct the wall of the femoral vein underlying the tumor, that was pathologically thickened, and communicated with the tumoral capsule. Although extremely rare, SS should be considered in the differential diagnosis of peripheral nerve sheath tumors, in particular if next to a large vein at the lower extremities.
INTRODUCTION
Synovial sarcoma (SS) represents ~5–10% of all the soft-tissue sarcomas (STS), and locates in the lower extremities in almost the 80% of cases [1]. The diagnosis of SS is based on the clinical evidences and on the radiological investigations (computed tomography (CT) and magnetic resonance imaging (MRi)), but not always these preoperative assessments are helpful to give a certain diagnosis of SS before the histological examination. The current treatment of SS is based on the surgical removal of the mass [2, 3] that should be performed, in case of tumors close to a nerve sheath and with an uncertain preoperative report, by a multispecialist team. The post-operative management is still an object of debate and the role of adjuvant treatments is actually controversial [4, 5].
We report a case of an adult patient with a crural SS, described as a femoral nerve shwannoma on the basis of the radiological investigations, which removal required the cooperation between neurosurgeons and vascular surgeons to attempt a good surgical outcome.
CASE REPORT
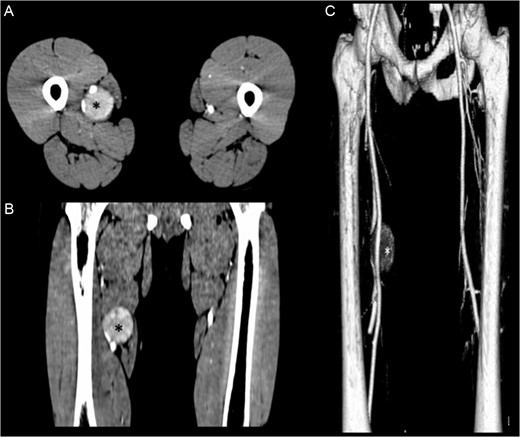
(A, B) Contrasted-enhanced CT scan shows a 4.1 × 4.3 × 4.6-cm mass (*) adjacent to the neurovascular bundle of the right thigh. (C) 3D reconstruction of the CT scan shows the displacement of the right femoral artery.
At the operation, after the exposure of the femoral neurovascular bundle, the mass appeared encapsulated and hardly dissectable from the femoral artery and vein. With the aim of an internal debulking of the mass, a 3-mm incision was performed on the tumoral capsule: after that we assisted to a massive arterial hemorrhage, which could not be controlled either with direct pressure on the lesion or with compression of the exposed proximal femoral artery. With the assistance of the vascular surgeon we isolated and temporary clamped the femoral artery at the mid inguinal point obtaining a reduction of the bleeding that allowed the tumor removal. Thus, we performed a wider opening of the capsule and completely removed a white-gray colored, gelatinous, friable mass. After the removal of the capsule we identified, in the proximal tumor bed, many arterial afferences arising from the femoral artery which were all coagulated with bipolar cauterization. Distally, the femoral vein showed a wide fissure communicating with the tumoral bed that was repaired with a patch graft. In the short time between the first incision on the tumoral capsule and the complete hemostasis after the tumor removal, the patient had lost an important blood volume with the halving of the hematocrit value and of the serum hemoglobin level and required many blood transfusions during the procedure and in the immediate post-operative care. The following post-operative period was regular, without any neurological or vascular complication, and the patient was discharged in the fourth day after surgery.
The histological examination of the samples described irregular tissue fragments of different dimensions (0.5–5 cm long), composed of proliferating spindle cells with moderate nuclear atypia and with a low mitotic rate, arranged in rough honeycombs with the plentiful interposition of capillary-shaped vascular structures. The immunohistochemical staining detected a focal reactivity for EMK (ecological momentary assessment) and Cytokeratin pool, with no reaction for actin, desmin, S100 protein, CD31, CD34 and CD99; the Ki-67 score was 8%. The cytogenetic study founded a typical molecular pattern of chromosomal t(X;18) translocation with expression of the SYT-SSX2 gene fusion products; these findings, together with the morphological and immunohistochemical patterns, were suggestive of a high-grade (G3—FNCLCC grading system) monophasic SS.
After the cytogenetic diagnosis, the patient did not receive any adjuvant therapy. A Positron emission tomography/CT scan, performed 3 months after the procedure, showed an area of high 18FDG uptake at the surgical bed, compatible with residual post-surgical inflammation. A following MRi investigation, performed 4 months after surgery, showed the surgical results with no evidence of local tumor recurrence.
DISCUSSION
SS is a relatively rare disease, which represents the 5–10% of all the STS. SS affects particularly the adolescent population and the young adults, with a median age at presentation of 30–35 years [1, 4, 6] and it usually locates at the lower extremities (80% of cases). An intravascular location of SS is also described but it is very uncommon, with only 10 well-documented cases reported in the literature, with the involvement of the large veins of the lower extremities and trunk [7] (Table 1). In our case, an involvement of the femoral vein by tumoral clots was not evident, even if the tumor capsule presented a communicating fissure with the underlying femoral vein (which wall appeared pathologically thickened).
| Author, year . | Age, sex . | Location . | Histology . |
|---|---|---|---|
| Miettinen et al. [16] 1987 | 34, female | Left femoral vein | Biphasic |
| Shaw and Lais [17] 1993 | 31, female | Inferior vena cava | Biphasic |
| Robertson et al. [7] 1998 | 34, female | Left superficial femoral vein | Biphasic |
| White et al. [18] 2005 | 56, female | Right external iliac vein | Biphasic |
| Tong et al. [19] 2006 | 32, female | Superior vena cava, right internal jugular vein, right proximal subclavian vein, protrusion into right atrium | Biphasic |
| Coen et al. [20] 2008 | 41, male | Right superficial femoral vein | Biphasic |
| Schoneveld et al. [21] 2012 | 32, female | Right common femoral vein | Monophasic |
| Tuncer et al. [22] 2012 | 16, male | Superior vena cava, right atrium | Biphasic |
| Wise et al. [23] 2012 | 41, female | Inferior vena cava, right hepatic vein | Monophasic |
| Schreiner et al. [24] 2015 | 20, female | Right superficial femoral vein to common iliac vein, profound femoral vein, great saphenous vein | Monophasic |
| Author, year . | Age, sex . | Location . | Histology . |
|---|---|---|---|
| Miettinen et al. [16] 1987 | 34, female | Left femoral vein | Biphasic |
| Shaw and Lais [17] 1993 | 31, female | Inferior vena cava | Biphasic |
| Robertson et al. [7] 1998 | 34, female | Left superficial femoral vein | Biphasic |
| White et al. [18] 2005 | 56, female | Right external iliac vein | Biphasic |
| Tong et al. [19] 2006 | 32, female | Superior vena cava, right internal jugular vein, right proximal subclavian vein, protrusion into right atrium | Biphasic |
| Coen et al. [20] 2008 | 41, male | Right superficial femoral vein | Biphasic |
| Schoneveld et al. [21] 2012 | 32, female | Right common femoral vein | Monophasic |
| Tuncer et al. [22] 2012 | 16, male | Superior vena cava, right atrium | Biphasic |
| Wise et al. [23] 2012 | 41, female | Inferior vena cava, right hepatic vein | Monophasic |
| Schreiner et al. [24] 2015 | 20, female | Right superficial femoral vein to common iliac vein, profound femoral vein, great saphenous vein | Monophasic |
| Author, year . | Age, sex . | Location . | Histology . |
|---|---|---|---|
| Miettinen et al. [16] 1987 | 34, female | Left femoral vein | Biphasic |
| Shaw and Lais [17] 1993 | 31, female | Inferior vena cava | Biphasic |
| Robertson et al. [7] 1998 | 34, female | Left superficial femoral vein | Biphasic |
| White et al. [18] 2005 | 56, female | Right external iliac vein | Biphasic |
| Tong et al. [19] 2006 | 32, female | Superior vena cava, right internal jugular vein, right proximal subclavian vein, protrusion into right atrium | Biphasic |
| Coen et al. [20] 2008 | 41, male | Right superficial femoral vein | Biphasic |
| Schoneveld et al. [21] 2012 | 32, female | Right common femoral vein | Monophasic |
| Tuncer et al. [22] 2012 | 16, male | Superior vena cava, right atrium | Biphasic |
| Wise et al. [23] 2012 | 41, female | Inferior vena cava, right hepatic vein | Monophasic |
| Schreiner et al. [24] 2015 | 20, female | Right superficial femoral vein to common iliac vein, profound femoral vein, great saphenous vein | Monophasic |
| Author, year . | Age, sex . | Location . | Histology . |
|---|---|---|---|
| Miettinen et al. [16] 1987 | 34, female | Left femoral vein | Biphasic |
| Shaw and Lais [17] 1993 | 31, female | Inferior vena cava | Biphasic |
| Robertson et al. [7] 1998 | 34, female | Left superficial femoral vein | Biphasic |
| White et al. [18] 2005 | 56, female | Right external iliac vein | Biphasic |
| Tong et al. [19] 2006 | 32, female | Superior vena cava, right internal jugular vein, right proximal subclavian vein, protrusion into right atrium | Biphasic |
| Coen et al. [20] 2008 | 41, male | Right superficial femoral vein | Biphasic |
| Schoneveld et al. [21] 2012 | 32, female | Right common femoral vein | Monophasic |
| Tuncer et al. [22] 2012 | 16, male | Superior vena cava, right atrium | Biphasic |
| Wise et al. [23] 2012 | 41, female | Inferior vena cava, right hepatic vein | Monophasic |
| Schreiner et al. [24] 2015 | 20, female | Right superficial femoral vein to common iliac vein, profound femoral vein, great saphenous vein | Monophasic |
In spite of its name SS do not have a synovial etiopathogenesis, and the term ‘synovial’ refers to the microscopical aspects of the neoplastic cells that are similar to the normal synovial tissue [4]. There are three histological subtypes of SS: the monophasic subtype, more common, composed of spindle cells; the biphasic subtype, composed of spindle cells associated to epithelial elements; and a poorly differentiated subtype, with diffused areas of necrosis and mitosis [1, 4]. At a biological level, the tumoral cells are characterized by a specific (X; 18) (p11; q11) translocation [8] with a resulting fusion gene (SYT—SSX1, SSX2, SSX4) that is an exclusive marker for the immunohistochemical diagnosis of SS, both primary and metastatic [6, 9].
The MRi usually shows a mass (frequently larger than 5 cm) located near a joint at the lower extremities with an inhomogeneous intensity on T2-weighted images [10] and isolated spotty calcifications, more evident on CT scan, that occur in ~30% of cases [11]. The MRi is also useful for the evaluation of some aggressive aspects of SS, such as an early contrast enhancement, or an involvement of the adjacent bone tissue and/or of the surrounding neuro-vascular structures [4].
The nuclear-imaging techniques are useful for a prognostic evaluation, as an FDG avidity bigger than 4.4 is associated to a higher risk of metastasis and local recurrence [12].
The treatment of SS is actually based on the surgical excision [2, 3], and the role of adjuvant radiotherapy and chemotherapy is still controversial. Many authors recommend the use of post-operative radiation therapy to obtain a better local control of the disease and to reduce the risk of local and distant recurrences, particularly in case of large tumors (>5 cm) that could not been completely removed [1, 4]. The role of chemotherapy is still object of debate: STS are usually chemoresponsive tumors and the use of ifosfamide, with or without doxorubicin, has not shown univocal results [4, 5].
The prognosis of SS patients depends on different factors and the female sex, a tumor size <5 cm, the biphasic histologic subtype, the age under 50 years at the diagnosis and negative resection margins after the tumor removal seem to be related to a better prognosis [13, 14] even without an adjuvant radiotherapy [15].
In a large series of SS patients [6], the recurrences, both local and distant, occur in a wide range of time (2–265 months , median time: 18 months) with an overall survival at 5 years of 68%. These results are similar to other SS studies [4, 11] and support the importance of a long-term follow-up for SS patients.
CONCLUSIONS
SS is a rare entity without a standardized therapeutic protocol. Many authors recommend considering SS in the differential diagnosis in cases of tumoral mass located at the lower extremities, especially if in proximity of a joint, in adolescent or in young adult patients. After our experience, and on the basis of the reported literature, we strongly suggest considering SS also in the differential diagnosis of peripheral nerve sheath tumor. Regardless, a multidisciplinar team that must be confident with this kind of STS is required for a better pre-surgical planning and for a wishful long-term follow-up.
Conflict of interest statement
None declared.



