-
PDF
- Split View
-
Views
-
Cite
Cite
Niklas Rommel, Oliver Bissinger, Andrea Rau, Thomas Muecke, Ectopic meningioma of the mandible in a 20-year-old woman: a case report and literature review, Journal of Surgical Case Reports, Volume 2017, Issue 3, March 2017, rjx047, https://doi.org/10.1093/jscr/rjx047
Close - Share Icon Share
Abstract
Ectopic meningiomas are a very rare tumor entity. We present a case of a meningioma arising in the mandible of a young woman and initially supposed to be a radicular cyst. Histopathological and immunhistochemical evaluation showed typical cell characteristics of a meningioma. Only six cases of ectopic meningiomas in the mandible have been described in the literature until now, mainly in women at an advanced age and with surgical removal of all tumors. For the first time, no surgical excision has been performed in this case and follow-up control after 12 months showed no significant progression or increasing clinical complaints. Hence, surgical removal seems non-urgent. In conclusion, unclear lesions of the jaws, even if they seem to be clear following diagnostics, should be evaluated by incisional biopsy and histopathological evaluation.
INTRODUCTION
Meningiomas are one of the most common neoplasms arising from cellular elements of the meninges, in particular, from arachnoid villi structures. Commonly, meningiomas are attached to the dura and grow in the cranial cavity or intraspinal region. However, ectopic locations of meningiomas cannot be excluded. In this report, we present a rare case of this special tumor entity in the mandibular bone of a young woman.
CASE REPORT
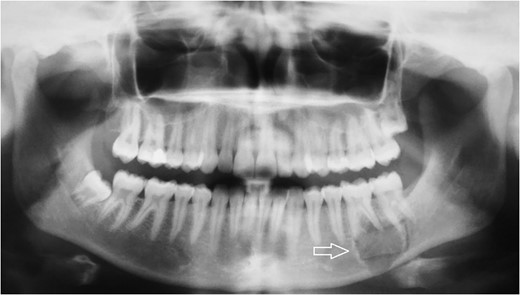
Radiologic diagnostics in two planes with panoramic X-ray. The panoramic radiograph showed a 2 × 1.8-cm radiolucent lesion of the left mandible. The lesion involved both apices of the first molar and the mesial apex of the second molar and extended to the caudal border of the mandibular bone.
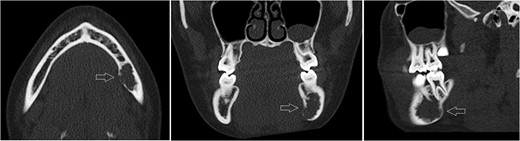
Axial, sagittal and coronar CT showing a 2 × 1 x 1.8-cm lesion in the left posterior mandible, its expansive and destructive character, and its penetration of the medial corticalis.
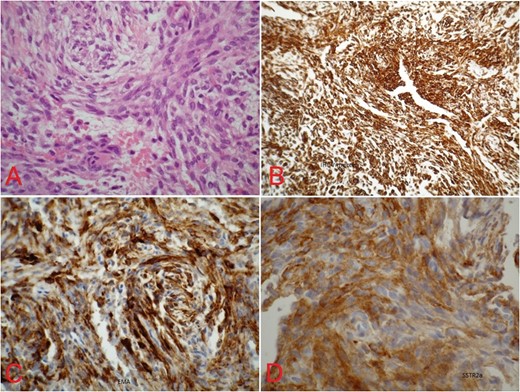
(A) Spindle-shaped cells arranged in cell cord and in whorls with no mitotic figures (hematoxylin–eosin, original magnification ×400). Immunhistochemical stains with immunopositive reaction for (B) vimentin (×100), (C) EMA (×400) and (D) somatostatin (×400).
DISCUSSION
Meningiomas are one of the most common tumor entities in the central nervous system, are generally benign and have their origin in the arachnoid villoid structures of the meningocytes. However, in rare cases, ectopic forms of this tumor entity can appear extracranially and extraosseously in the head and neck region. With respect to the jaws, we have found only eight cases including two meningiomas of the maxilla [1, 2] and six meningiomas of the mandible [3–7] in the current literature. We have found the seventh case of an extracranial meningioma of the mandible in a young woman who presented with a cystoid-like lesion in the left mandibular bone and no specific clinical symptoms. Because of the absence of typical radiographic features, no clear diagnosis was possible either with a panoramic X-ray or 3D imaging. However, the role of CT is seen significantly to assess the relationship between the tumor and the bony surfaces and to exclude potential malignancy [8].
Several hypotheses have been proposed for the occurrence of extracranial meningiomas, including extradural enclosing of arachnoid cell nests during embryogenesis, ectopic migration, and the development of arachnoid cells in combination with the peripheral nerves, or metaplasia of the mature peripheral nerve sheath cells or progenitor cells [8]. However, ectopic meningiomas have also been postulated to be mesenchymal tumors that arise from multipotential mesenchymal cells, particularly if no associations to the cranial nerves are apparent [9]. In the head and neck region, this tumor entity is often associated with cranial nerves and, therefore, is considered to be derived from ectopic arachnoid tissue present around these nerves [3]. Therefore, the origin of the tumor arising in our patient seems to be the perineural cells of the mandibular nerve. However, ectopic arachnoid cells within the mandibular bone as a source of tumor growth cannot be excluded.
The histopathologic and immunhistochemical features of ectopic meningiomas are seen similar to those of their more frequent intracranial counterparts [4, 8]. The general histologic diagnosis of meningiomas describes spindle-shaped cells that can be arranged in whorls, rosettes and interconnecting fascicles. Mitotic figures or atypia is generally rare, and psammom bodies might be present [4]. Because of the differential diagnosis from other tumor entities of peripheral nerve origin, an immunhistochemical analysis is useful or even necessary. In meningiomas, the positive expression of EMA, desmoplakin, somatostatin and vimentin is a characteristic feature [3, 4, 10] and demonstrates the epithelial and mesenchymal patterns of cells within this tumor.
Epidemiologically, ectopic meningiomas are slightly more frequent in females with a ratio of 1:1.2, i.e. ~55% [8]. The average age of patients is 43.4 years, whereby females are older (48.7 years) than males (36.9 years) [8]. On closer consideration of extracranial meningiomas of the mandibular bone, all previously described cases in the current literature were women with an average age of 45.7 years. In our case, the gender was the same, but the age of the patient was, at 20 years, considerably younger than the average.
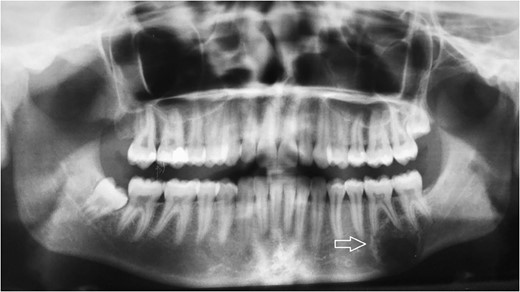
Follow-up panoramic X-ray imaging at 12 months after initial diagnosis showing no significant progression of the tumor lesion.
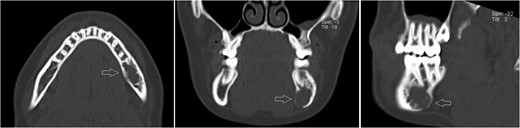
Likewise to panoramic X-ray, follow-up CT after 12 months showing no significant progression of the lesion.
The conclusion of this case is that unclear lesions of the jaws, even if they seem to be clear following diagnostics, should be evaluated by incisional biopsy and histopathological evaluation.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
All authors have read and approved all versions of the manuscript and its content. No ethical approval has been required. The authors would like to thank Theresa Jones for the final language correction of the manuscript.
FUNDING
None.
REFERENCES
Author notes
These authors contributed equally to the manuscript.



