-
PDF
- Split View
-
Views
-
Cite
Cite
Tomke Cordts, Amir K. Bigdeli, Leila Harhaus, Christoph Hirche, Thomas Kremer, Ulrich Kneser, Volker J. Schmidt, Pyoderma gangrenosum following complex reconstruction of a large-scale lower limb defect by combined Parascapular and latissimus dorsi flap, Journal of Surgical Case Reports, Volume 2017, Issue 1, January 2017, rjw241, https://doi.org/10.1093/jscr/rjw241
Close - Share Icon Share
Abstract
A female patient with a critical soft tissue defect after elective knee replacement surgery was transferred to our department for reconstruction. As wounds were rapidly progressing, necrotizing fasciitis was initially suspected but eventually ruled out by histopathological analysis. A 50 × 15 cm defect was then reconstructed by means of a combined Parascapular and latissimus dorsi flap before, a couple days later, the patient developed tender pustules and ulcers involving the flap as well as the donor site. Attempts of excising necrotic areas not only continued to fail but seemed to worsen the patient's wound and overall condition. Eventually, pyoderma gangrenosum (PG) was diagnosed and local and systemic therapy was initiated but treatment proved to be challenging and insufficient at first. Being an extremely aggressive disease, early diagnosis is crucial and PG should always be suspected when rapidly progressive ulceration on surgical sites is observed.
INTRODUCTION
Pyoderma gangrenosum (PG) is a rare neutrophilic skin disease characterized by rapidly progressing painful, necrotic ulceration and typically affects patients in their third to sixth decade of life [1, 2]. It most frequently involves lower extremities and remains a diagnosis of exclusion, as there are no specific laboratory or histopathologic findings [3]. In most cases, an underlying systemic disorder, such as Inflammatory bowel disease, Hepatitis C, Rheumatoid arthritis, hematologic or lymphoproliferative disorder, can be identified [4] but a substantial amount of cases remain elusive. In 25–50%, skin lacerations by trauma or surgery will eventually trigger new lesions (pathergy phenomenon) [5] and thereby exacerbate progression even further. This renders PG a malicious and challenging disorder, especially in the surgical patient.
CASE REPORT
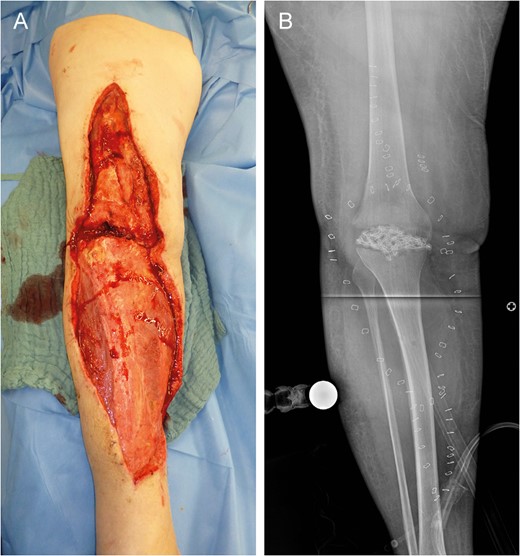
Left lower limb tissue defect on admission (A) and corresponding X-ray (B).
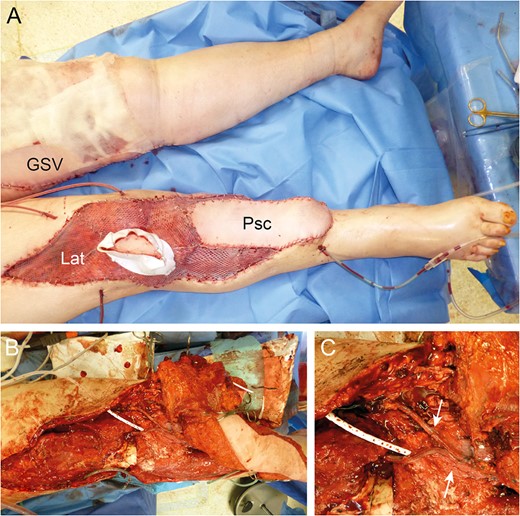
Reconstruction by combined Parascapular (Psc) and latissimus dorsi (Lat) flap with GSV donor site (A). Intraoperative images with pedicle anastomoses (arrows) (B and C).
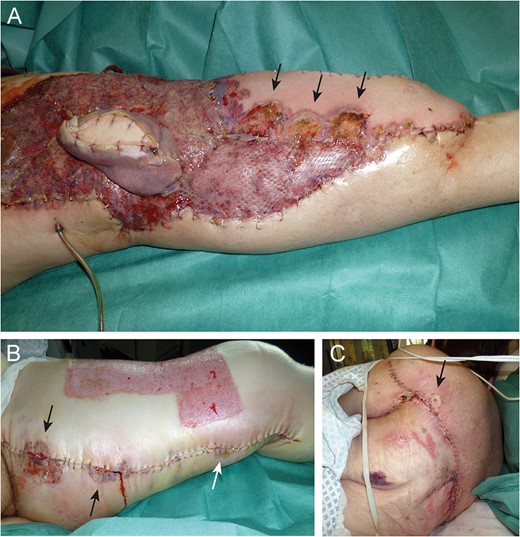
Eleven days after tissue coverage: suppurative, necrotic ulcers (arrows) on flap (A) and donor sites (B and C).
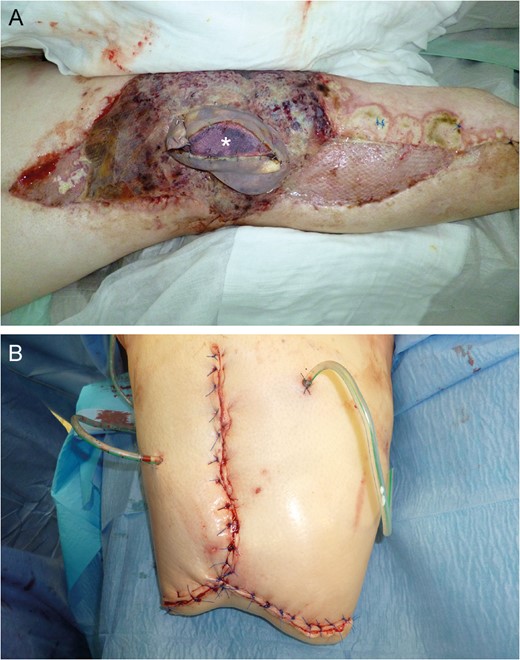
Near-total flap loss on Day 22. Note the livid discoloration of the monitor island (asterisk) indicating severe tissue hypoxemia (A). Above knee amputation site (B).
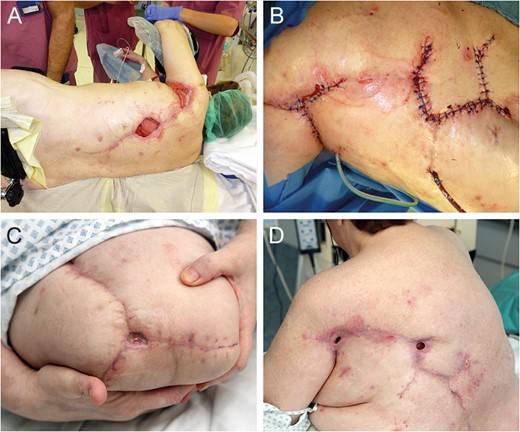
Flap donor site coverage by rhomboid transposition flap (A and B). State on demission (C and D).
DISCUSSION
When developing PG, patients that originally agreed to standardized, low risk and routinely performed procedures may suddenly find themselves in a potentially life threatening situation. For the treating surgeon, it is much more likely to attribute postoperative ulcerative necrosis to an unspecific infectious process than taking PG into consideration. Additionally to being extremely rare [2], it lacks specific serologic or histologic markers and has a broad list of differential diagnoses [3].
A usual approach to postoperative wound necrosis is surgical excision. But in PG, these attempts may accelerate disease progression even further. Attributable to the pathergy phenomenon, which is present in 25–50% of cases [3], lesions are likely to develop at any body region where skin is traumatized—donor sites of skin grafts, surgical incisions and even puncture sites for invasive blood pressure monitoring and central lines when the patient is admitted to ICU. Confirmation of diagnosis is helpful, but the treating physician may suddenly find himself in a very delicate situation, as almost all manually performed procedures are likely to be contraindicated. In our case, the effects were so severe that amputation of the left leg had to be performed, but after a few days, ulcers started to appear at the amputation site as well.
Once diagnosis is confirmed by a dermatologist, empirical systemic treatment needs to be initiated as targeted or specific therapy is usually not available. An exception may be if associated with a systemic disorder, e.g. inflammatory bowel disease. Control of the underlying condition usually results in control of the skin lesions [6] but in PG following surgery, no such option is available. A combination of local wound care, topical and/or systemic therapy is recommended and addresses both pathophysiological components—a systemic inflammatory and a wound component. PG lesions are almost universally painful, so proper pain management needs to be assured as well [3]. First line systemic therapy is based exclusively on immunosuppression with corticosteroids and/or cyclosporine. Generally, an initial prednisone dose of 1–2 mg/kg/day is recommended to achieve control of acute disease. Cyclosporine at 3–6 mg/kg/day can be used in patients with contraindications to corticosteroids (e.g. prior history of intolerance, psychiatric disorder, osteoporosis) and to achieve rapid control of acute and aggressive disease [7]. Topical therapy involves local application of corticosteroids, tacrolimus and cyclosporine to the inflamed border of the ulcer rather than its base [3].
Substantial data are lacking and, in general, the outcome after PG is considered to be unpredictable [4]. A follow-up of 44 cases found a remission rate of 44%, whereas 56% of patients still required continuing therapy [1]. Additionally, relapse rate seems to be high with 66–70% in a review of 26 cases [8].
PG is rare but should always be included as a differential diagnosis when rapidly progressive ulceration on surgical sites is observed. Over 80 years after its first description, the disease still remains poorly understood and its appearance is especially challenging in patients requiring large-scale tissue reconstruction. The treating surgeon must know and properly identify the underlying disease as early as possible to hopefully limit its dreadful consequences.



