-
PDF
- Split View
-
Views
-
Cite
Cite
J.P. Regan, J.T. Casaubon, E. Genelus-Dominique, Synchronous invasive ductal carcinoma in encapsulated papillary ductal carcinoma, Journal of Surgical Case Reports, Volume 2016, Issue 8, August 2016, rjw141, https://doi.org/10.1093/jscr/rjw141
Close - Share Icon Share
Abstract
Encapsulated papillary ductal carcinoma (EPC) of the breast is a rare form of cancer with defining histopathology of encapsulation. These lesions are typically indolent but may rarely have concomitant, synchronous invasive lesions.
This report details a 56-year-old black female who presented with a palpable left breast mass. Adenosis with focal fibrous and ductal hyperplasia characteristics were found on core needle biopsy. Excisional biopsy showed EPC with invasive components. A simple mastectomy was performed and a second lesion was identified as invasive ductal carcinoma.
EPC typically has good prognosis and a low incidence of invasion. The risk increases in the presence of a second, synchronous lesion as in our case. Management is typically performed with breast conserving methods; however, missing a second lesion is possible. This report provides an overview of the literature and discussion of the role of MRI in preoperative workup.
Introduction
Encapsulated papillary ductal carcinoma (EPC) of the breast is a rare subtype of ductal carcinoma. It makes up ~0.5–1% of all breast carcinomas. It is characterized by malignant transformation of the breast epithelium supported by a fibrovascular stalk. In particular, these lesions are surrounded by a fibrous capsule that separates the lesion from the breast stroma. These lesions are known to have good prognosis secondary to the low risk of invasion and can be considered in situ lesions. If encapsulating myoepithelial cells are lacking, the lesion is considered invasive, yet still carries a favorable prognosis [1–5].
Treatment of these lesions is compatible with its ductal counterpart and based on conventional tumor staging. EPC has shown to be effectively treated with local excision and adjuvant hormonal therapy with tamoxifen for 5 years duration. Breast conserving therapy is optimal if indicated [1, 2].
Case Presentation
A 56-year-old black female presented to the surgical clinic with a palpable left breast mass that had grown over several months. She denied nipple discharge, breast retraction or family history of malignancy. Physical exam revealed a palpable, subareolar mass without skin changes or asymmetry, and no lymphadenopathy. Her mammogram revealed a 5.4 × 3 cm lesion behind the nipple with Breast Imaging-Reporting and Data System (BI-RADS) 5 classification. An ultrasound indicated a 2.2 × 2 cm lobulated hypoechoic mass with BI-RADS 4 classification (Figs 1–3).
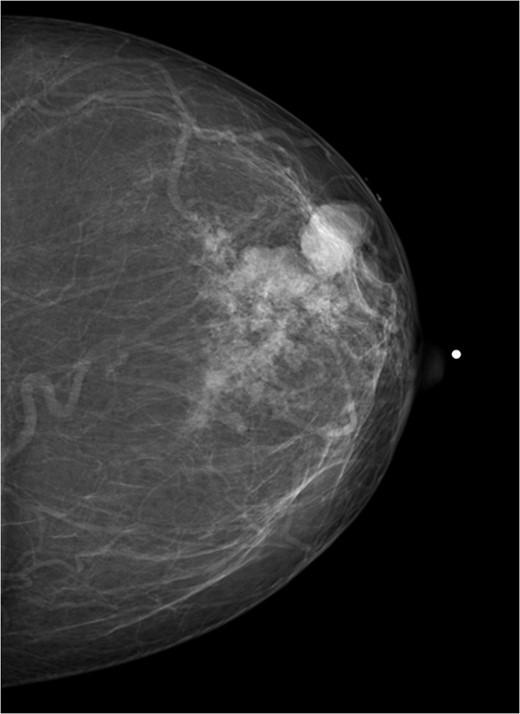
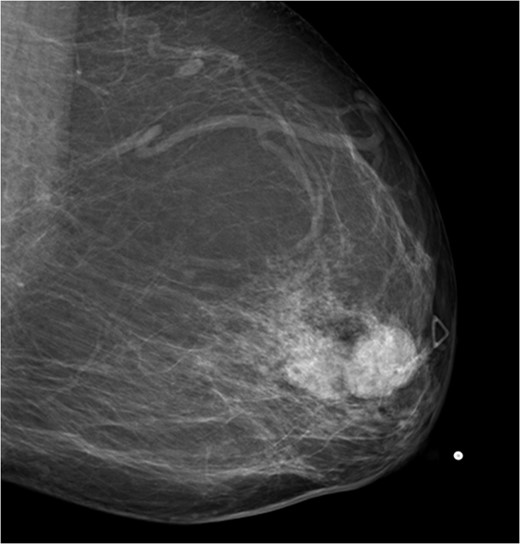
Mammogram mediolateral view of left breast showing lesion posterior to nipple BI-RADS 5.
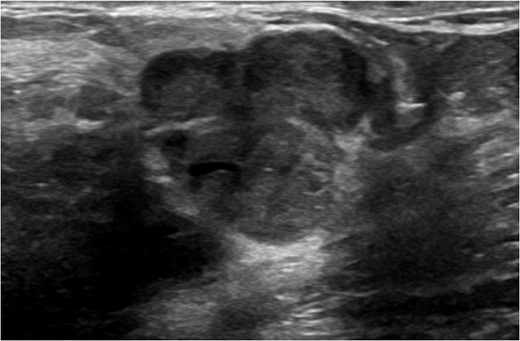
Ultrasound of left breast showing multilobulated lesion BI-RADS 4.
An ultrasound-guided, core needle biopsy was performed. Pathology indicated adenosis with focal fibrosis and ductal hyperplasia. A decision was made to perform excisional biopsy based on the aggressive pathologic appearance.
The patient underwent excisional biopsy which revealed a 3 × 2.4 × 1 cm EPC negative for lymphovascular invasion with positive staining for spindle cells, suggestive of encapsulation with myoepithelium. The biopsy was noted to be strongly ER/PR positive and HER2 negative with positive margins.
The patient was then sent for MRI of the breast to detail the extent of the remaining mass. This revealed an ill-defined mixed linear and fine nodular enhancing tumor 6.5 × 4.6 × 4.8 cm in size with BI-RADS 5 (Figs 4–7).
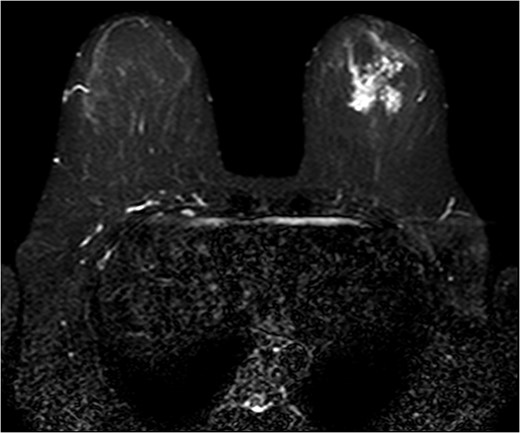
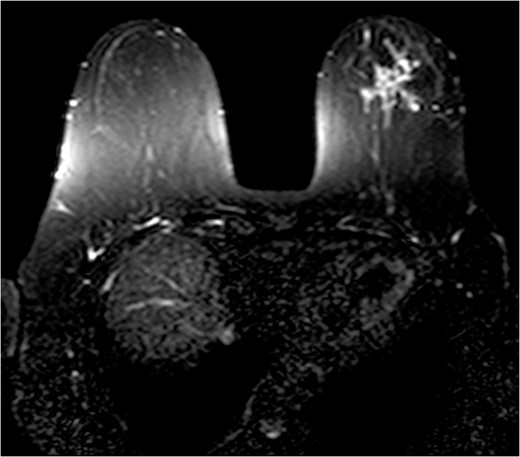
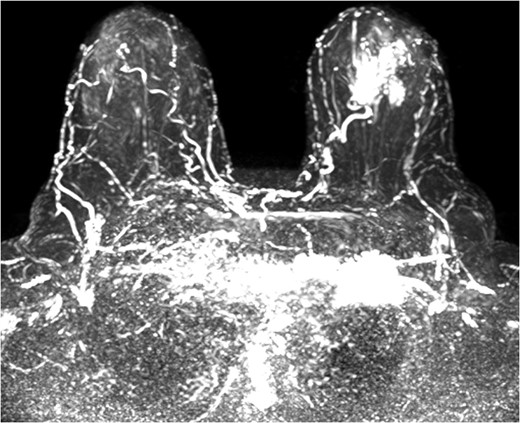
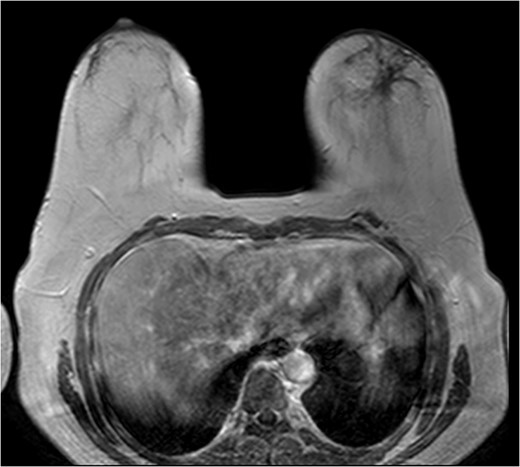
Due to the location of the residual lesion posterior to the nipple and its large size, simple mastectomy with sentinel lymph node biopsy was performed with immediate breast reconstruction. Breast conservation surgery was not considered secondary to the location and size. Histology showed a focus of ductal carcinoma in-situ (DCIS) with micropapillary growth and a secondary synchronous invasive ductal carcinoma 0.7 × 0.4 × 0.3 cm adjacent to the lesion. Four lymph nodes were excised and all negative for invasive characteristics. Staging indicates a T1N0M0, Stage I lesion.
Samples were sent for oncotyping to determine the risk of recurrence and need for adjuvant therapy which revealed a Breast Cancer Recurrence Score of 0. Patients with a Recurrence Score of 0 had an average rate of recurrence of 3% [6]. Based on the stage and oncotyping, the patient will undergo 5 years of Tamoxifen therapy alone. She is currently doing well and continues to have close follow-up.
Discussion
EPC is a rare form of breast cancer which is classified as intracystic or solid. Solid variants are characterized by mucin production and neuroendocrine features. EPC, when totally encapsulated, is classified as an in situ lesion. The lesion is considered invasive if an incomplete capsule is found.
This subtype is typically found in postmenopausal females between the ages of 60–70. Patients typically do not have a family history and present with a palpable breast mass without lymphadenopathy [1–3].
Screening mammogram will identify these lesions as round and well-circumscribed. Ultrasound will show a multilobulated solid mass typically 1–2 cm in size in the retroareolar region. Core needle biopsy under ultrasound guidance is effective for tissue diagnosis [4, 5]. False negatives may occur due to sampling of the capsule instead of the lesion itself.
The role of MRI in identifying the extent of breast carcinoma is widely reviewed. EPC has been noted to have multifocal hyperintensities at T1 weighted imaging. Contrast MRI will reveal enhancement of the cystic wall, septa and mural nodules [4].
Though these lesions have been frequently described, their subtypes were never thoroughly investigated which resulted in many patients undergoing modified radical mastectomies [2]. It was not until the mid-1980s when one author noted three distinct subtypes of these lesions which are shown to have indolent courses [7]. A retrospective case series revealed that these lesions are effectively treatment with breast conserving surgery and hormonal therapy alone [2].
These lesions have a 30% chance of coexisting synchronous lesion [1–3]. There is little discussion in the literature regarding whether these secondary lesions are missed on conservative surgical management. In our patient, the decision to perform a simple mastectomy was due to the large size of the lesion and its subareolar location. The synchronous lesion was small and may have been missed if breast conservative treatment was performed.
With an increased risk of a second, invasive lesion, the clinician should be wary and recognize the risk when tumor pathology reports a papillary carcinoma of the breast. Considering the effective use of MRI in evaluation of tumor extent [8–10], the clinician should not hesitate to use this tool for the diagnosis of lesion size or presence of synchronous lesions when planning surgical excision.
Acknowledgement
There are no acknowledgement.
Conflict of Interest Statement
None declared.



