-
PDF
- Split View
-
Views
-
Cite
Cite
Soh Nishimoto, Masato Kinoshita, Yuko Miyazaki, Kenichiro Kawai, Masao Kakibuchi, Lymphoedema of the penis and scrotum as a sequela of chronic skin infection, Journal of Surgical Case Reports, Volume 2016, Issue 7, July 2016, rjw127, https://doi.org/10.1093/jscr/rjw127
Close - Share Icon Share
Abstract
A Japanese male patient presented with an enormously disfigured penis and scrotum. The penis was swollen and distorted rightward, and the skin was hard and lumpy. The patient had had a subdermal abscess for 6 years. The current condition was considered secondary lymphoedema of the penis and scrotum resulting from chronic skin infection. Wide excision of the affected area with bilateral inguinal lymph node dissection were performed. The degloved penile shaft and scrotum were covered with skin grafts, and a satisfactory result was obtained.
Introduction
Lymphoedema is a condition of abnormal lymphatic fluid retention, and the cause of lymphoedema varies. Congenital lymphoedema is a state of lympho-vascular malformation. Obstruction of the lymphatic drainage system may lead to secondary lymphoedema. Acquired lymphoedema of the penis and scrotum, except for iatrogenic cases, is not common in our clinical practice. We encountered a man with an enormously disfigured penis and scrotum. After excluding several diagnoses, chronic skin infection was assumed to be the cause. Wide local excision with bilateral superficial inguinal lymph node dissection and reconstruction led to a satisfactory outcome.
Case Report
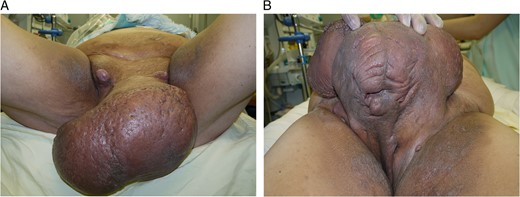
A 48-year-old Japanese man (body mass index: 34.5 kg/m2) presented (Fig. 1). Approximately 6 years previously, he had recognized a small area of induration with purulent discharge on his scrotum. Gradually, his scrotum and penis had swollen without pain. According to his statement, he was able to urinate normally despite the penile enormity. His swollen penis was distorted rightward, and the glans and external urethral orifice could not be identified. The skin was thick and rough, and several dimples with purulent discharge were seen. Enlarged lymph nodes were palpable in the bilateral inguinal regions. Laboratory data showed a high white blood cell count (161.2 × 102/μl) and C-reactive protein (3.9 mg/dl). There was no sign of immunocompromised status.

A 48-year-old man presented with a disfigured penis and scrotum.
Methicillin-sensitive Staphylococcus aureus, Prevotella oralis and Peptostreptococcus asaccharolyticus were positive in the purulent discharge. DNA detection by polymerase chain reaction for Chlamydia trachomatis was negative in both the urine and the abscess discharge.
The patient kept no pets, and he had no particular record of travelling abroad. There was no record of neoplasm, trauma, surgery or irradiation. He denied a familial presence of any oedematous conditions.
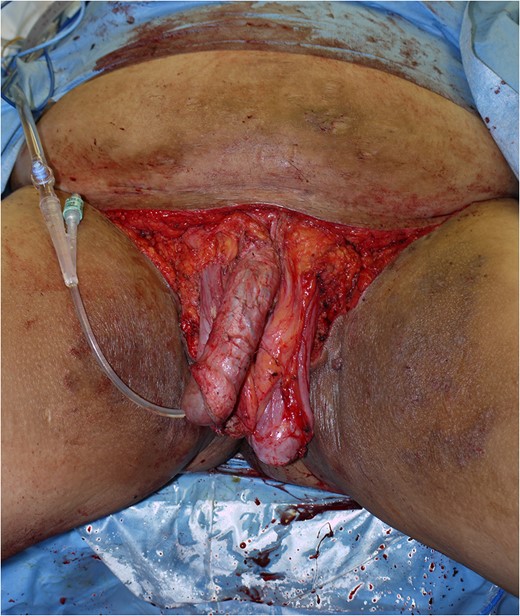
To reduce the excessive volume of the genitals, a surgical operation was performed (Fig. 2 A and B). After general anaesthesia was induced, a skin incision was first made on the penis to explore the glans. There were several dimples on the penis, so it was difficult to find the location of the external urethral tunnel. The glans was exposed and seemed intact, and a urethral catheter was then inserted without difficulty. Bilateral inguinal dissection of the superficial lymph nodes was performed, and the spermatic cords were identified and isolated at this time. A dorsal incision on the pachydermatous penile skin was done down to the areolar connective tissue on the deep fascia. The scrotal skin was also excised above the cremasteric fascia (Fig. 3). The resected specimen measured 25 × 30 × 20 cm and weighed 3.6 kg (Fig. 4).
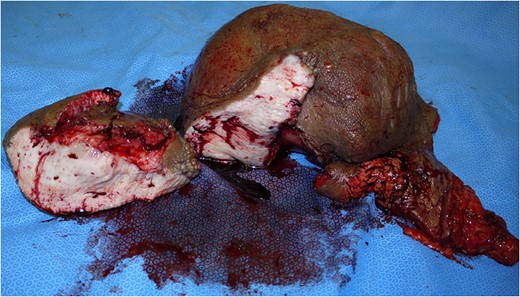
The resected specimen and cross-section. It measured 25 × 30 × 20 cm and weighed 3.6 kg.
Split skin grafts were harvested from the bilateral thighs. The penile shaft was covered with a sheet graft, placing the suture line spirally to avoid scar contracture, and the testes were wrapped with a meshed skin graft (Fig. 5).

The penile shaft was covered with a sheet skin graft. The scrotum was covered with a meshed skin graft.
Histopathologically, massive fibrosis from the dermis to the subdermal tissue with fibroblast proliferation was seen. No sign of filarial parasites was identified. Lymph node swelling was concluded to be a reactive condition due to chronic inflammation.
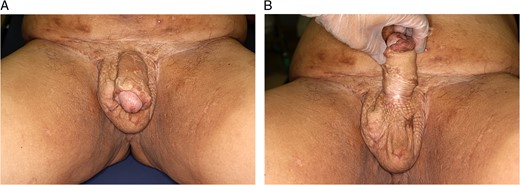
In the early postoperative period, serous exudate was observed, but no albuminous supplementation was needed. The white blood cell count and C-reactive protein levels normalized shortly after the surgery. The patient was satisfied with the result both cosmetically and functionally (Fig. 6 A and B).
Discussion
Lymphoedema is a condition involving the chronic collection of lymphatic fluid. Acquired obstruction of the lymphatic system may cause secondary Lymphoedema. Classic elephantiasis tropica, which is caused by obstruction of the lymphatic system with filarial parasites, is well known and not rare in certain geographic areas. Lymphogranuloma venereum is caused by Chlamydia trachomatis infection. Tuberculosis and neoplastic tumours also may be the causes of lymphoedema. Surgeries or irradiation, by disrupting the lymphatic system, may block lymphatic flow and cause the development of chronic lymphoedema.
In this report, we presented a case with an enormous lymphoedematous condition of the penis and scrotum, which is quite rare in our community. It was not easy to evaluate the aetiology. Resident inhabitant bacteria were detected in the purulent discharge, and we concluded that chronic local infection, or hidradenitis suppurativa, developed into obstruction of the lymphatic flow. Although the association of chronic pyogenic infection with genital lymphoedema has been reported [1–6], it is not widely recognized. There have been several case reports, though none from Eastern Asia.
Although various conservative remedies have been suggested for hidradenitis suppurativa, the definitive therapy is wide surgical excision, as Lane [7] adopted.
There are two lymphatic systems in penis:[8] the superficial system and the deep system. The superficial system drains the prepuce and skin of the penis, and it flows into the superomedial zone of the superficial inguinal nodes. The deep system drains the glans, runs beneath the deep fascia and flows both directly into the pelvic nodes and to the superficial inguinal nodes. These anatomic structures can explain the discrepancy between the severely involved penile skin and the intact glans, as observed in our patient. Because circumferential excision of the penile skin above the deep fascia does not interfere with the deep lymphatic system [2, 9, 10], secondary penile lymphoedema is unusual.
There may be discussion about our decision to perform bilateral inguinal lymph node dissection. We assessed that a bacterial infection had spread to the inguinal lymphatic system, so we planned to dissect the superficial regional lymphatic systems to control the infection. No leg lymphoedema has been seen after the surgery.
Conflict of interest statement
None declared.



