-
PDF
- Split View
-
Views
-
Cite
Cite
Timothy James Pengilley Bray, Hoskote Chandrashekar, Jeremy Rees, Ailbhe Burke, Ashirwad Merve, Stefanie Thust, Venous infarction mimicking a neoplasm in spontaneous intracranial hypotension: an unusual cause of Parinaud's syndrome, Journal of Surgical Case Reports, Volume 2016, Issue 3, March 2016, rjw037, https://doi.org/10.1093/jscr/rjw037
Close - Share Icon Share
Abstract
We present a case of longstanding, undiagnosed spontaneous intracranial hypotension (SIH) with an acute presentation of Parinaud's syndrome, in whom serial imaging demonstrated development of a midbrain mass. The patient was ultimately diagnosed with tumefactive venous infarction secondary to SIH. However, this patient underwent a brainstem biopsy, which in retrospect may have been avoidable. This case demonstrates the imaging features of tumefactive venous infarction in SIH and highlights the risk of misinterpretation as a neoplasm with potentially catastrophic consequences.
INTRODUCTION
Spontaneous intracranial hypotension (SIH) classically presents with orthostatic headache, particularly exacerbated by laughing, coughing and Valsalva manoeuvre [1]. Imaging features include subdural haematomas, thickening and enhancement of the pachymeninges, downward displacement of the brain and enlargement of the pituitary gland [1]. However, SIH may occur atypically with vague clinical signs, and a number of cases are occult [2].
When imaging is performed, isolated changes in brainstem morphology can be difficult to interpret. SIH characteristically causes ‘sagging’ of the brainstem with flattening of the pons, but in the absence of other signs, this anatomical distortion may be overlooked or misinterpreted.
We present a patient with longstanding undiagnosed SIH with an acute presentation of Parinaud's syndrome, in whom serial imaging demonstrated development of a midbrain mass. This case demonstrates the imaging features of tumefactive venous infarction in SIH and highlights the risk of misinterpretation as a neoplasm with potentially catastrophic consequences for biopsy.
CASE REPORT
A 58-year-old man presented to community hospital services with a 3-year history of bifrontal headache and associated neck pain. His symptoms usually worsened towards the evenings, when straining or exerting himself physically, and showed some degree of improvement overnight. There had been no response to analgesia prescribed by a general practitioner. A computed tomography and subsequent magnetic resonance imaging (MRI) scan performed 2 years earlier (in November 2012) were reported as normal.
Leading up to his hospital presentation, the patient had begun to experience subtle blurring of vision, needing to give up driving as a result. A specialist ophthalmology assessment revealed impaired ocular motility featuring an upward gaze palsy and nystagmus, provoked by upgaze with exotropia in the right eye. Central vision was essentially preserved and there was no significant refractive error.
In September 2014, the patient was referred to quaternary neurology services suffering progressive difficulty in walking, loss of balance and poor concentration, in addition to headache and oculomotor symptoms. Examination of the cranial nerves confirmed bilateral upward gaze palsy, a constricted, unreactive left pupil and convergence-retraction nystagmus consistent with Parinaud's syndrome. Visual acuity and fundoscopy were normal in both eyes, trigeminal and facial nerves intact bilaterally. Peripheral nerve examination revealed mild, bilateral dysmetria, and an extensor plantar reflex on the right side.
The patient underwent a series of four MRI scans between 2012 and 2015. The first MRI performed in November 2012, which had erroneously been reported as normal, shows downward brainstem displacement (Fig. 1a). Of note, subdural collections and meningeal enhancement were absent. A second MRI performed in early September 2014 revealed a moderately expansile lesion occupying the posterior aspect of the midbrain (Fig. 1b).
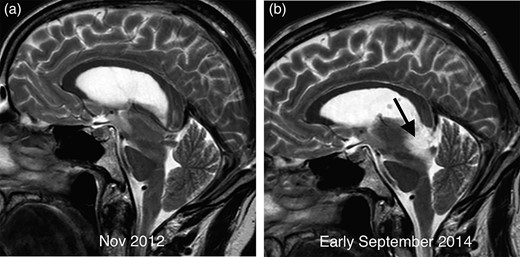
Comparative sagittal T2 imaging performed 2 years apart in demonstrating sagging of the brainstem with elongation of the midbrain and marked flattening of the pons surface. The scan from early September 2014 (b) demonstrates a new T2 hyperintense mass in the midbrain (arrow) compared with the earlier image (a).
A third MRI performed in late September 2014 during an acute admission for worsening visual symptoms demonstrated interval enlargement of the midbrain mass (Fig. 2a and b) featuring acute petechial haemorrhage (Fig. 2c). There was no restricted diffusion or contrast enhancement at any time point. Sagging of the brainstem was evident on both studies in 2014, unchanged from the initial scan performed 2 years earlier.
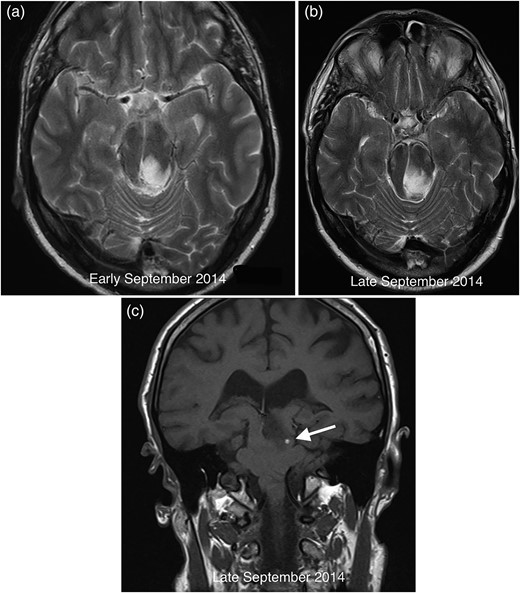
Comparative axial T2 images demonstrating growth of the midbrain mass between early (a) and late (b) September 2014. The coronal T1 image from September 2014 (c) reveals petechial haemorrhage (arrow) in the lesion with corresponding susceptibility artefact on gradient echo imaging (not shown).
Although the possibility of intracranial hypotension was considered during clinicoradiological discussion, the growing lesion raised concern for a neoplasm and a consensus decision was made to perform a stereotactic biopsy.
After biopsy, the specimen showed profuse haemorrhage, thought to be secondary to the procedure itself. The specimen showed no evidence of malignancy, but reactive features with a small volume of fresh haemorrhage and engorged vessels. A GFAP stain revealed severe gliosis, with spider-shaped reactive glia (Fig. 3).
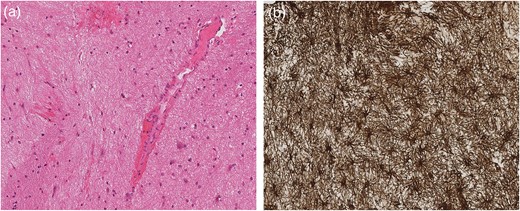
Grey matter parenchyma sample (a) consistent with brainstem biopsy showing prominent vessels and a small volume of haemorrhage. The GFAP stain (b) within the same sample confirms severe gliosis featuring spider-shaped reactive glia.
Following a transient deterioration in balance following the biopsy, the patient clinically improved with subtotal resolution of his oculomotor symptoms. This was mirrored by a mild improvement in the degree of brainstem sagging on a follow-up MRI from 2015, further supporting a diagnosis of SIH (Fig. 4). The patient declined a lumbar puncture and spinal imaging to assess for signs of a CSF leak.
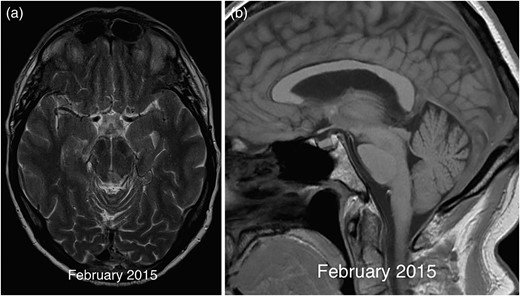
Axial T2 image in 2015 (a) demonstrating resolution of the midbrain mass except for a small biopsy scar. The corresponding sagittal T1 (b) image shows a mild improvement in the degree of brainstem sagging with less marked flattening of the pons compared with previous imaging.
DISCUSSION
This case highlights the risk of misinterpreting tumefactive venous infarction in undiagnosed SIH as tumour. The authors would like to bring this dilemma to the attention of the surgical community, who may be consulted to perform biopsy. If venous ischaemia presents as an expansile lesion, the combination of mass effect and the absence of restricted diffusion may prevent diagnosis as an infarct, because in arterial infarction, diffusion restriction is a hallmark feature in the acute phase. Slowly progressive infarction, as evidenced by serial imaging and the presence of gliosis in this case, may be especially difficult to recognize. In the absence of associated imaging findings, it can also be challenging to recognize a sagging brainstem and distinguish this from a hindbrain malformation, whereby certain malformations can be associated with tumour [3, 4]. Indeed, controversy exists around a recently reported type of malformation (proposed to arise secondary to early anteroposterior transformation at the diencephalic-mesenchephalic junction) [3], which was considered by other authors to be identical to features in SIH [4].
Venous infarction most commonly occurs in the context of venous sinus thrombosis, imaging signs of which include parenchymal swelling, CSF space effacement and a reduction in ventricular calibre [5]. Diffusion-weighted imaging can to some extent ‘classify’ patients according to the predominant mechanism of cerebral oedema—diffusivity is increased in vasogenic oedema and reduced in cytotoxic oedema. In some cases, both types of oedema may be present simultaneously [6]. Parenchymal enhancement has been reported in the context of cerebral venous thrombosis [7] but is in general non-specific. Haemorrhage is observed in a minority of patients with venous sinus thrombosis and is typically cortical with subcortical extension [5].
In SIH patients, the key feature appears to be vasogenic oedema in the majority of cases, and lesions are most commonly found in the brainstem [8]. The increased diffusivity and mass-like behaviour of these lesions are probably due to vasogenic oedema as a result of venous stagnation [8]. This venous stagnation may occur as a result of venous engorgement compensating for the loss of CSF volume as per the Monro-Kellie hypothesis [9] or due to impaired drainage of the deep venous system [8].
There are a number of findings in this case that point to the correct diagnosis. Swelling and mass effect are well-known features of venous ischaemia and should prompt suspicion in the presence of coexisting brainstem distortion [3]. The relatively rapid (within 2 weeks) enlargement and development of acute haemorrhage within the lesion are unusual for brainstem neoplasms, which are commonly of low to intermediate grade.
In summary, tumefactive venous midbrain infarction represents a rare but ultimately diagnosable complication of SIH, which poses a substantial bleeding risk on biopsy. Patients may not necessarily make a spontaneous recovery, but they can suffer a life-threatening brainstem compromise that may only be reversible by treating the underlying SIH.
Finally, to the authors' best knowledge, ours is the first reported case of SIH-related venous infarction presenting as classic Parinaud's syndrome. Parinaud's comprises a supranuclear palsy of vertical gaze resulting from damage to the mesencephalon [10]. The syndrome encompasses gaze paralysis (particularly vertical movements), convergence disorders and unreactive pupils. The critical lesion is thought to lie in the efferent paths of the rostral interstitial nuclei of the medial longitudinal fasciculus [10]. The major causes of Parinaud's syndrome are brain tumours, multiple sclerosis and arterial ischaemic infarcts; our case suggests that venous infarction might also be considered in this list.
CONFLICT OF INTEREST STATEMENT
None declared.



