-
PDF
- Split View
-
Views
-
Cite
Cite
Solveig Aalstad Jonasson, Dag Eirik Jøssang, Rune Haaverstad, Øystein Wendelbo, Gustav Pedersen, Arteriovenous fistula of the groin in a drug abuser with endocarditis, Journal of Surgical Case Reports, Volume 2016, Issue 2, February 2016, rjw001, https://doi.org/10.1093/jscr/rjw001
Close - Share Icon Share
Abstract
Intravenous drug abusers commonly develop endocarditis due to injection of particulate matter that can cause endothelial damage to the valves. The frequent need to access the venous system can result in vascular traumas with potential complications including arteriovenous (AV) fistulas. Here, we present the case of an intravenous drug abuser with endocarditis and an unusually large AV fistula in the groin. The patient was successfully operated for endocarditis. However, the AV fistula was at the time not acknowledged. The combination of ileofemoral vein thrombosis and a large AV fistula led to pulmonary septic embolism and life-threating, right-sided heart failure. Computed tomography scan did not reveal the AV fistula, but suspicion was raised. Ultrasound diagnosed and revealed the magnitude of the AV fistula, and the patient was treated with a minimally invasive percutaneous technique.
INTRODUCTION
Intravenous drug users (IVDUs) commonly experience complications caused by infections and vascular traumas—particularly right-sided endocarditis with Staphylococcus aureus, infected haematomas, pseudoaneurysms and thromboemboli. An AV fistula can occur due to a pseudoaneurysm or as a complication of injections. Drug abusers are often hospitalized due to infections and should be examined for underlying complications.
CASE REPORT
A 37-year-old white male with a 20-year history of intravenous drug abuse was admitted after experiencing fever and chills for the past month, with progressive worsening. He had been treated with antibiotics twice for suspected pneumonia. Acute delirium onset led to hospitalization. The patient had no history of chest pain or haemoptysis, but cough and fever were reported.
At admission, the patient appeared septic and had a systolic heart murmur. Clinical examination revealed the following: respiration rate, 32/min; blood pressure, 132/64 mmHg; pulse, 122/min; and temperature, 39.8°C. Table 1 presents the results of admission laboratory tests. Chest X-ray showed left-sided pneumothorax and pleural effusion, and findings consistent with pulmonary congestion and pneumonia. Thoracic computed tomography (CT) revealed multiple septic emboli in the lungs, pleural effusion and a small left-sided pneumothorax. Within 24 h post-admission, S. aureus grew in repeated blood cultures.
| Laboratory test . | Result . | Normal range . |
|---|---|---|
| C-reactive protein | 176 mg/l | <5 mg/l |
| Haemoglobin | 7.8 g/dl | 13.4–17.8 g/dl |
| MCV | 81 fl | 82–98 fl |
| Leukocyte count | 29.1 × 109/l | 3.5–11.0 × 109/l |
| Thrombocyte count | 106 × 109/l | 145–348 × 109/l |
| Creatinine | 86 µmol/l | 60–105 µmol/l |
| Sodium | 117 mmol/l | 137–145 mmol/l |
| Potassium | 3.5 mmol/l | 3.5–5.0 mmol/l |
| Troponin T | 111–95 ng/l | <15 ng/l |
| proBNP | 1000 pmol/l | <10 pmol/l |
| D-dimer | 3.05 mg/l | <0.5 mg/l |
| International normalized ratio spontaneous | 1.9 | <1.1 |
| Laboratory test . | Result . | Normal range . |
|---|---|---|
| C-reactive protein | 176 mg/l | <5 mg/l |
| Haemoglobin | 7.8 g/dl | 13.4–17.8 g/dl |
| MCV | 81 fl | 82–98 fl |
| Leukocyte count | 29.1 × 109/l | 3.5–11.0 × 109/l |
| Thrombocyte count | 106 × 109/l | 145–348 × 109/l |
| Creatinine | 86 µmol/l | 60–105 µmol/l |
| Sodium | 117 mmol/l | 137–145 mmol/l |
| Potassium | 3.5 mmol/l | 3.5–5.0 mmol/l |
| Troponin T | 111–95 ng/l | <15 ng/l |
| proBNP | 1000 pmol/l | <10 pmol/l |
| D-dimer | 3.05 mg/l | <0.5 mg/l |
| International normalized ratio spontaneous | 1.9 | <1.1 |
| Laboratory test . | Result . | Normal range . |
|---|---|---|
| C-reactive protein | 176 mg/l | <5 mg/l |
| Haemoglobin | 7.8 g/dl | 13.4–17.8 g/dl |
| MCV | 81 fl | 82–98 fl |
| Leukocyte count | 29.1 × 109/l | 3.5–11.0 × 109/l |
| Thrombocyte count | 106 × 109/l | 145–348 × 109/l |
| Creatinine | 86 µmol/l | 60–105 µmol/l |
| Sodium | 117 mmol/l | 137–145 mmol/l |
| Potassium | 3.5 mmol/l | 3.5–5.0 mmol/l |
| Troponin T | 111–95 ng/l | <15 ng/l |
| proBNP | 1000 pmol/l | <10 pmol/l |
| D-dimer | 3.05 mg/l | <0.5 mg/l |
| International normalized ratio spontaneous | 1.9 | <1.1 |
| Laboratory test . | Result . | Normal range . |
|---|---|---|
| C-reactive protein | 176 mg/l | <5 mg/l |
| Haemoglobin | 7.8 g/dl | 13.4–17.8 g/dl |
| MCV | 81 fl | 82–98 fl |
| Leukocyte count | 29.1 × 109/l | 3.5–11.0 × 109/l |
| Thrombocyte count | 106 × 109/l | 145–348 × 109/l |
| Creatinine | 86 µmol/l | 60–105 µmol/l |
| Sodium | 117 mmol/l | 137–145 mmol/l |
| Potassium | 3.5 mmol/l | 3.5–5.0 mmol/l |
| Troponin T | 111–95 ng/l | <15 ng/l |
| proBNP | 1000 pmol/l | <10 pmol/l |
| D-dimer | 3.05 mg/l | <0.5 mg/l |
| International normalized ratio spontaneous | 1.9 | <1.1 |
Based on these findings, right-sided native valve endocarditis (NVE) due to S. aureus was suspected. Following established guidelines [1, 2], the patient was started on empiric antibiotic treatment with 2 g cloxacillin 6 times daily and 320 mg gentamicin once daily. The patient was considered clinically unstable and was transferred to a university hospital the day after admittance.
Transthoracic echocardiogram (TTE) and transoesophageal echocardiogram (TEE) revealed aortic endocarditis with vegetations, aortic valve destruction, Grade 4 aortic insufficiency and small mitral insufficiency, but no signs of mitral valve vegetations. Infective endocarditis was diagnosed based on the modified Duke criteria [1, 2], as the patient met two major criteria (S. aureus growth from two blood cultures and endocardial involvement evidenced on echocardiogram) and three minor criteria (intravenous drug use, fever and vascular phenomena with septic pulmonary infarcts). However, there were no signs of right-sided endocarditis to explain the septic embolization to the lungs.
CT scan revealed right-sided thrombi in the ileofemoral vein segment. The patient exhibited severe cardiac decompensation despite intensive medical treatment, and thus underwent open heart surgery with biological aortic valve insertion 1 week after hospitalization. Postoperatively, the patient exhibited pleural effusion despite repeated pleural fluid drainage, and large amounts of pericardial fluids, generalized oedema, and clinical signs of right ventricle cardiac failure with pulmonary congestion.
Two weeks postoperatively, reinvestigation of previous CT scans showed rapid contrast filling in the inferior caval vein (Fig. 1a) as well as the right common iliac vein (Fig. 1b), almost simultaneously with the abdominal aorta. Recent CT scans showed dissolution of most of the right-sided, ileofemoral deep vein thrombus. Right groin palpation revealed fremissement. Doppler ultrasound demonstrated an unusually large arteriovenous (AV) fistula localized between the cranial part of the superficial femoral artery and the femoral vein. Flow in the fistula was estimated as 11.2 l/min.
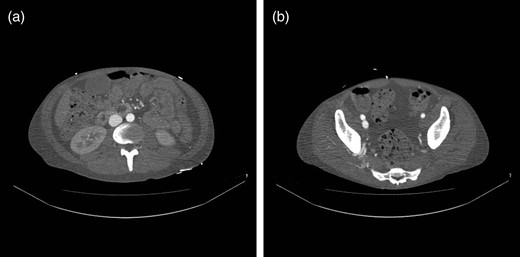
(a) CT scan demonstrating rapid contrast filling of the vena cava. (b) CT scan demonstrating contrast filling of the right common iliac vein.
Endovascular treatment of the AV fistula was performed in the following day. The contralateral femoral artery was accessed, angiography showed the fistula (Fig. 2a) and a stent-graft (Fluency, Tempe AZ, USA) was implanted in the cranial part of the right superficial femoral artery, covering the fistula (Fig. 2b). Treatment eliminated the arterial phase contrast filling in the veins. The patient recovered rapidly and was discharged 1 month later, after a total hospital stay of almost 8 weeks. Intravenous antibiotic treatment (cloxacillin and ciprofloxacin) continued until discharge. After discharge, he received oral trimethoprim sulfamethoxazole for another 4 weeks. At last follow-up, he showed no signs of recurrent infections, but was still intermittently abusing drugs.
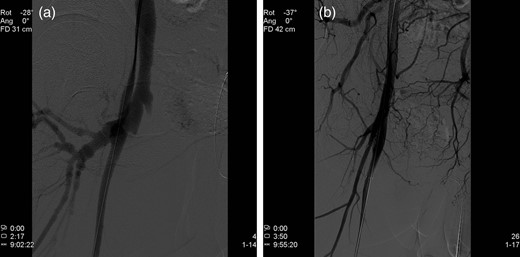
(a) Angiography demonstrating an AV fistula between the right superficial femoral artery and the femoral vein. (b) A stent-graft is implanted in the cranial part of the right superficial femoral artery, sealing the AV fistula.
DISCUSSION
In this case, 20 years of intravenous drug abuse had obliterated all accessible superficial veins of the patient's extremities, and thus vascular access was obtained using deep veins in the groin.
The endocarditis diagnosis was confirmed by both TTE and TEE. S. aureus is a common pathogen affecting heart valves. Guidelines state that initial therapy should include penicillinase-resistant penicillin or vancomycin, depending on local methicillin-resistant Staphylococcus aureus prevalence [1]. Infectious Diseases Society of America guidelines indicate that aminoglycoside use for 3–5 days may be considered [2]. Recommended therapy duration is 4–6 weeks. Later clinical examination of the groin revealed fremissement and re-evaluation of the CT scans showed rapid contrast filling of the vena cava, which are both highly suspicious of an AV fistula that were not initially recognized. CT scanning cannot determine AV fistula magnitude and clinical relevance. However, Doppler ultrasound can both diagnose an AV fistula and estimate the magnitude of the flow. Here, we found extremely high flow (11.2 l/min), leading to life-threatening, right-sided heart failure. Subsequent CT scans showed near dissolution of the ileofemoral thrombosis with simultaneous increase of pulmonary emboli. This was probably due to the high fistula flow flushing thrombi from the ileofemoral vein segment, embolizing to the lungs, and thereby increasing right ventricle pressure and leading to right ventricle heart failure.
Vascular prosthesis implantation in patients with active infection is traditionally contraindicated. Open surgery for a groin AV fistula in an IVDU carries significant risk. Therefore, a minimally invasive treatment was preferred. Treatment of the fistula was done percutaneously under local anaesthesia with implantation of a stent-graft with subsequently rapid recovery of the patient. This method is usually not preferred in IVDUs due to the risk of infecting the stent-graft. The experience with stent-grafts for treating mycotic arterial aneurysm [3] can be used to support the use of stent-graft in the present case. The benefit of avoiding open surgery outweighs the risk of infecting the stent-graft.
In this patient, early detection and treatment of the AV fistula might have prevented a potentially critical postoperative condition. Doppler ultrasound enabled diagnosis and flow estimation, and endovascular treatment carrying minimal risk was applied.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
Author notes
Both authors contributed equally.



