-
PDF
- Split View
-
Views
-
Cite
Cite
Nkwam Nkwam, Bethan Johnson, Alvaro Bazo, Tom A. McCulloch, Gurminder S. Mann, Inflammatory myofibroblastic tumour of the urinary bladder managed with partial cystectomy: a case report & literature review, Journal of Surgical Case Reports, Volume 2016, Issue 11, November 2016, rjw181, https://doi.org/10.1093/jscr/rjw181
Close - Share Icon Share
Abstract
Inflammatory myofibroblastic tumour (IMT) is a rare neoplasm which can affect many different organs throughout the body. We report the case of a 62-year old female presenting with visible haematuria found to have IMT of the urinary bladder exhibiting anaplastic lymphoma kinase-1 gene rearrangement, initially managed with local resection and then definitively with partial cystectomy.
INTRODUCTION
Inflammatory myofibroblastic tumour (IMT) – also known as inflammatory pseudotumour (IPT) – is a rare neoplasm described in multiple locations throughout the human body, including the lung – where it was first described by Bunn in 1939 – and larynx [1, 2]. It has also been reported in the kidneys, prostate, ureter and epididymis [3–5]. The malignant potential is unknown and often locally aggressive, presenting in a similar way to transitional cell carcinomas of the bladder. We report the case of IMT of the urinary bladder in a female patient presenting with haematuria.
CASE REPORT
A 62-year-old female presented with a 5-day history of visible haematuria. Clinical examination was unremarkable. Full blood count analysis revealed a haemoglobin of 58 g/L prompting transfusion of 3 units of packed red cells. An ultrasound scan (USS) identified an abnormal soft tissue mass arising from the superior aspect of the urinary bladder with vascular flow within it, raising the suspicion of a malignant neoplasm (Fig. 1).
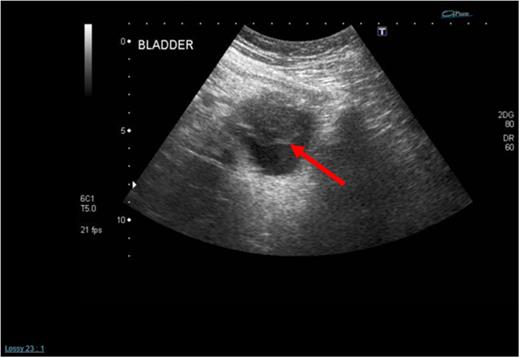
Initial admission USS of the urinary bladder demonstrating a solid mass within it (see red arrow).
An urgent transurethral resection of the bladder tumour (TURBT) was performed which demonstrated a solid 4 cm tumour in the anterior wall of the urinary bladder. Resection was complete and tissue sent for histopathological analysis. A staging computed tomography (CT) scan post-op confirmed the absence of any locally advanced disease, lymphadenopathy or distant metastases (Fig. 2). Initial histopathological analysis reported the presence of muscle-invasive Grade 3 transitional cell carcinoma of the bladder with sarcomatoid change (G3pT2), but a supplementary report soon after confirmed positive smooth muscle actin (diffuse and tram track), anaplastic lymphoma kinase-1 (ALK1), focally positive CK7, AE1/3, weakly positive focally S100, and negative CD34, desmin, EMA, caldesmon, MNF116, p63. The sample was described as a highly cellular but monotonous appearing spindle-cell proliferation within the bladder, set in lightly myxoid stroma with scattered inflammatory cells. Overall, the features were in keeping with an IMT. Fluorescence in situ hybridization for ALK1 fusion was undertaken and studies of 103 cells, using the Cytocell ALK, chromosome 2p23-specific, dual colour probe showed that ALK rearrangement was present, confirming a diagnosis of IMT.
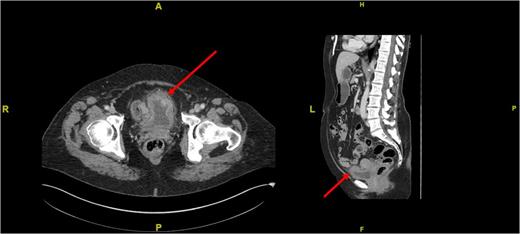
Cross-sectional (left) and sagittal (right) CT images of the abdomen and pelvis demonstrating a thickening of the anterior bladder wall at the site of the first TURBT (see red arrows).
We embarked on close endoscopic surveillance and six weeks later a rigid cystoscopy revealed a 6 cm solid recurrence at the previous resection site. This was completely resected and histopathological analysis again confirmed IMT recurrence with ALK1 gene rearrangement. Due the low reported incidence of metastases bladder conservation was the preferred option but since it recurred so quickly we opted for partial cystectomy. A further pelvic magnetic resonance imaging (MRI) performed 4 weeks post-op demonstrated 22 mm × 10 mm irregular area with associated increased enhancement involving the left anterolateral wall of the bladder which in keeping with the site of the primary resection and no evidence of locally advanced disease (Fig. 3).
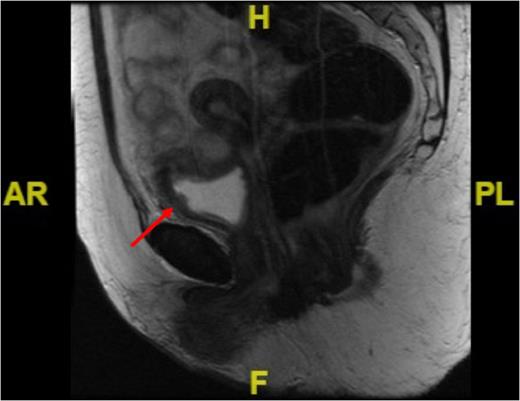
Sagittal MRI image of the pelvis showing irregularity in the anterolateral bladder wall at the site of resection (see red arrow).
Our patient recovered well following open partial cystectomy and histology revealed the complete resection of a 32 mm × 35 mm piece of bladder wall with a 12 mm central ulcerated dimple consisting of residual IMT with ALK1 positivity. At 6-month clinic review she remains well without recurrence.
DISCUSSION
In cases of IMT histological diagnosis is not always easy. Appearances can be mistaken for sarcoma due to the spindle-cell appearances with inflammatory infiltration at microscopy. These tumours, however, do not have atypical mitotic cells and often stain positive for ALK1 expression. This has also been reported in cases of IMT of the urinary bladder and can be used to differentiate between IMT and sarcomas. Hirsch and colleagues (2006) reported on 27 cases of pseudosarcomatous myofibroblastic proliferations of the urinary tract in which 10 had ALK1 positivity and 6 of these were negative for ALK gene rearrangement, unlike our case which was positive [6].
There are incidences of IMT of the bladder related to infection as described in a 38-year old woman presenting with dysuria and fever in a 4 cm IMT was found. Management was conservative with local resection and her symptoms settled post-op [7]. Diagnosis of IMT can be challenging affecting men, women and children alike. Lecuona and colleagues (2012) reported the case a 3-year old boy who presented with visible haematuria and IMT managed with partial cystectomy. They advocated close clinical follow-up due to the unknown biological behaviour of these tumours [8].
Gofrit and colleagues published work describing the significance of IMT in patients with a history of bladder cancer [9]. They retrospectively reviewed the cases of 809 patients with bladder cancer in which 16 went on to develop IMT with 12 patients (75%) developing recurrence, nine patients (56%) tumour progression, and six patients (37.5%) dying of bladder cancer. They concluded that a diagnosis of IMT in a patient previously diagnosed with bladder cancer is associated with a high risk of tumour recurrence, progression, and cancer-related mortality, hence, close endoscopic surveillance is advised.
Although referred to as a benign tumour, IMT has been reported to be locally aggressive and muscle invasive often requiring multiple transurethral resections or partial cystectomies. A review of 44 patients with IMT by Kovach and colleagues in 2006 described a range of treatment options patients had – complete resection, incomplete resection, observation, or chemotherapy and/or radiation [10]. Five patients had adjuvant chemotherapy or radiotherapy after surgery (14%) and, of note, there were three reported cases of local recurrence after partial resection (8%) all occurring in patients without adjuvant chemo- or radiotherapy. Our patient was treated with partial cystectomy in which margins were clear and there are no signs of recurrence 6 months on. This option was favourable to the patient as it offered a radical approach with bladder preservation.
Complete aggressive surgical resection appears to be the key to avoiding recurrence. In patients having bladder-sparing techniques close endoscopic surveillance is advised. Recurrence of Bladder IMT has not been reported in the literature following complete surgical resection, therefore, we recommend partial cystectomy followed by close endoscopic surveillance as a viable, safe, bladder-sparing option in the management of IMT.
CONFLICT OF INTEREST STATEMENT
None declared.



