-
PDF
- Split View
-
Views
-
Cite
Cite
Moosa Kunhi, Sachin Sanagar, N. Jagadeesh, Vadanattathil P. Gangadharan, Anand Kumar., Pushpa Mahadevan, A case of primary intracardiac yolk sac tumour with extracardiac extension, Journal of Surgical Case Reports, Volume 2016, Issue 11, November 2016, rjw187, https://doi.org/10.1093/jscr/rjw187
Close - Share Icon Share
Abstract
Primary cardiac tumour is a rare entity as secondaries in the heart are more common. A 2-year-old child was having repeated respiratory tract infection with poor oral intake and poor activity for 3 months. His symptoms progressed from New York Heart Association (NYHA) Class II to IV. On evaluation he had an intracardiac mass with extracardiac extension. Emergency tumour excision under deep hypothermic circulatory arrest was performed with provisional diagnosis of sarcoma. But Serum markers, histopathological examination and immunohistochemistry confirmed diagnosis of yolk sac tumour. Postoperative recovery was uneventful and the child was receiving adjuvant chemotherapy. Extensive literature review revealed only four cases of primary intracardiac yolk sac tumour published till date. Our case report is unique, in that intracardiac tumour had extracardiac extension by infiltration through right atrial wall. Previous four reports mention purely intracardiac mass.
Introduction
Primary cardiac tumours are a rare entity whose incidence according to surgery and autopsy reports is 0.3–0.7% of all cardiac tumours [1]. Germ cell tumours are benign or malignant neoplasms arising from primordial germ cells which can be gonadal or extragonadal in origin. Primary intracardiac yolk sac tumour is extremely rare, only four cases being reported till date. We report probably fifth case.
Case Report
A 2-year-old male child was brought to hospital with respiratory distress since last 2 weeks. He was having repeated respiratory tract infection since last 3 months along with poor oral intake and poor activity. His symptoms progressed from New York Heart Association (NYHA) Functional Class II to IV over last 2 weeks. On investigation at another hospital he was diagnosed with intracardiac mass having extracardiac extension. Parents brought the child to our hospital for further management.
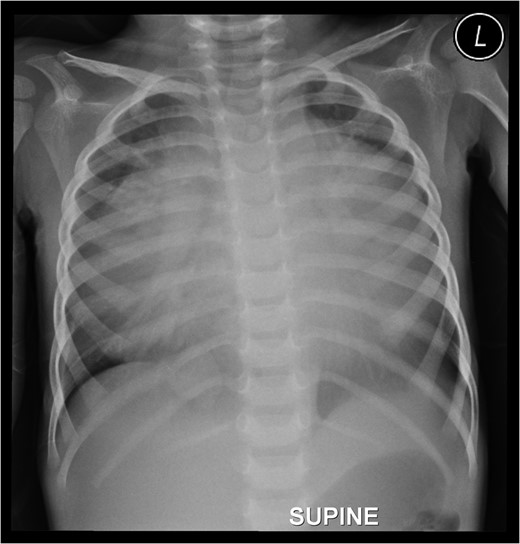
Preoperative chest roentgenogram showing cardiomegaly and massive pericardial effusion.
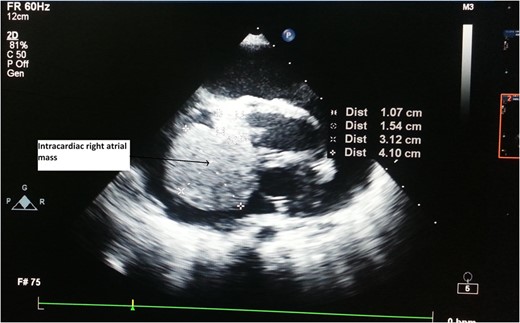
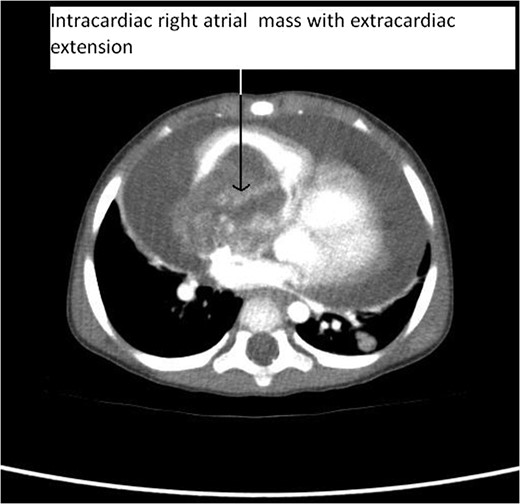
CT Thorax showing intracardiac right atrial mass with extracardiac extension.
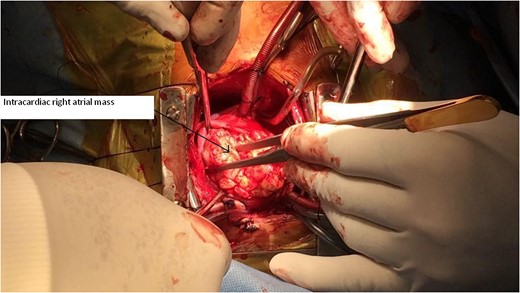
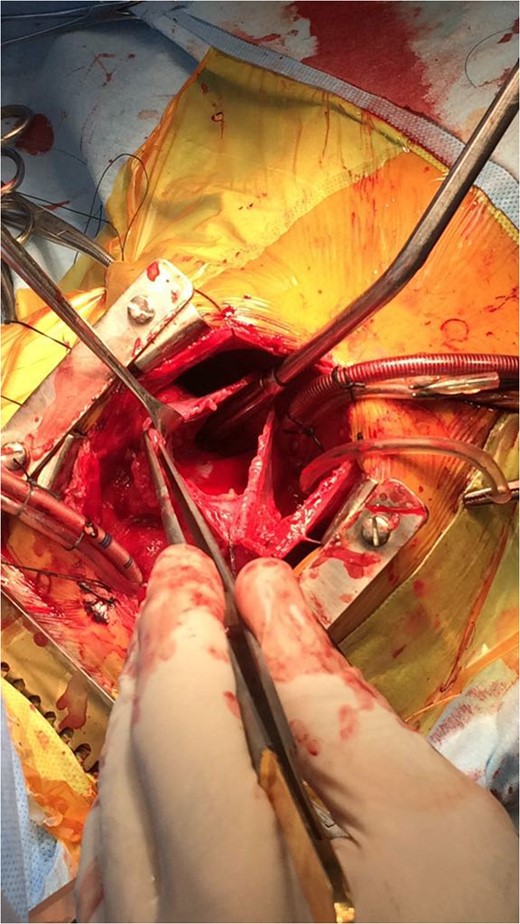
Serum markers on day of surgery were Alpha Feto Protein (AFP) 131 146 ng/ml (normal range 0–20 ng/ml), Beta subunit of Human Chorionic Gonadotropin (beta hCG) 0.5 mIU/ml (normal range < 2 mIU/ml), Lactate Dehydrogenase (LDH) 1231 U/L (normal range 0–850 U/L)
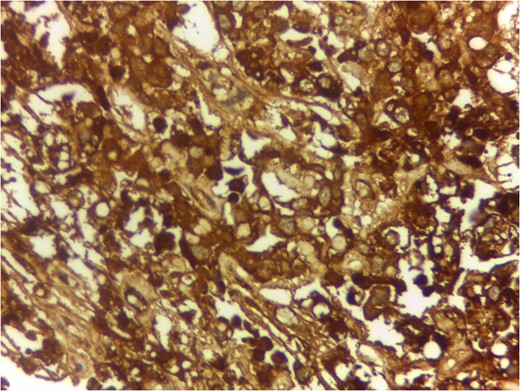
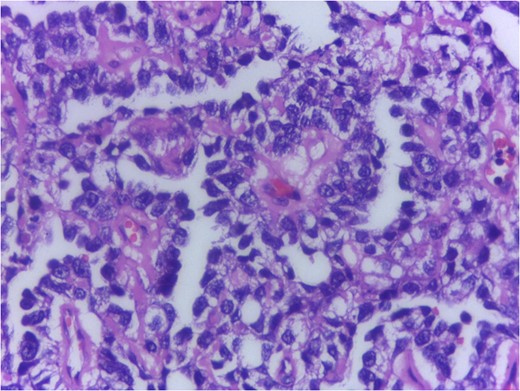
Serum markers, histopathological examination and IHC all three confirmed diagnosis of yolk sac tumour.
Postoperatively Serum AFP was elevated to 830 900 ng/ml. Early chemotherapy was started with Bleomycin, Etoposide and Cisplatin (BEP) regimen. The child was tolerating chemotherapy well. After second cycle of chemotherapy Serum AFP level dipped to 2025 ng/ml showing good response to chemotherapy. Ultrasonography of abdomen pelvis and testis was normal prior to chemotherapy. We will review patient after each chemotherapy cycle and in long term for prognosis and recurrence if any.
Discussion
Primary cardiac tumour is rare. Only 25% of primary cardiac tumours are malignant. Survival rate for malignant primary cardiac tumours without surgical resection at 9–12 months is only 10%. Sarcomas constitute 75% of malignant primary cardiac tumour. Germ cell tumour is rare. Germ cell tumours are due to abnormal differentiation of foetal germ cells that arise from the foetal yolk sac. Normal migration of these germ cells may cause gonadal tumours, i.e. ovary and testis, whereas abnormal migration produces extragonadal tumours. Most cardiac germ cell tumours are teratomas. Yolk sac tumour is extremely rare. Surgical resection followed by chemotherapy is preferred treatment for yolk sac tumour. Only four cases of primary intracardiac yolk sac tumour are published till date in medical literature. Those are listed in Refs [2–5]. Three other cases of yolk sac tumour are reported but they were extracardiac being in pericardial cavity [6]. We are probably reporting fifth case of primary intracardiac yolk sac tumour.
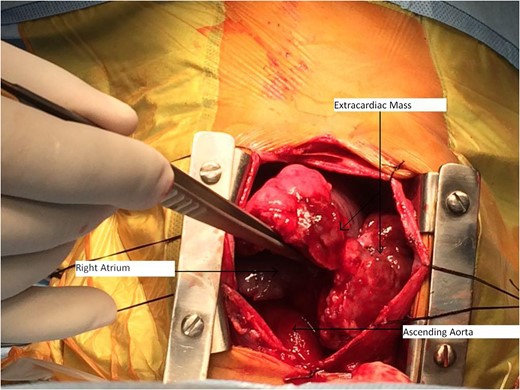
Our case report is unique, in that intracardiac tumour had extracardiac extension by infiltration through right atrial wall. Previous four reports mention purely intracardiac mass. The rarity in the literature of such pathological occurrence makes our case report very unique.
In conclusion, as far as the rarity of the tumour is considered, measures such as thorough preoperative evaluation and preparation, intraoperative decision making and surgical expertise, proper postoperative chemotherapy are must for better outcome.
Acknowledgements
The authors would like to acknowledge Dr Ami Maria Emmanuel, MBBS, MD, Sr. Registrar—Pathology Lakeshore Hospital and Research Centre, Kochi, Kerala, India.
Supplementary Material
Supplementary material is available at the Journal of Surgical Case Reports online.
Conflict of Interest Statement
None declared.



