-
PDF
- Split View
-
Views
-
Cite
Cite
Yardesh Singh, Shamir O. Cawich, Leyrone Olivier, Thivy Kuruvilla, Fawwaz Mohammed, Vijay Naraysingh, Pancreatic pseudocyst: combined single incision laparoscopic cystogastrostomy and cholecystectomy in a resource poor setting, Journal of Surgical Case Reports, Volume 2016, Issue 11, November 2016, rjw176, https://doi.org/10.1093/jscr/rjw176
Close - Share Icon Share
Abstract
Laparoscopic cystogastrostomy is a well-accepted minimally invasive modality to treat pancreatic pseudocysts. There has been one prior report of cystogastrostomy via single incision laparoscopic surgery (SILS) in which specialized instrumentation and access platforms were used.
We report the challenges encountered in a low resource setting with the SILS approach to drainage using only standard laparoscopic instruments. To the best of our knowledge this is the second report of SILS cystogastrostomy and the first to be performed in a resource poor setting without specialized instruments or platforms.
INTRODUCTION
Between 5% [1] and 14% [2] of patients develop a pancreatic pseudocyst after an episode of acute pancreatitis. Although the majority of pseudocysts resolve spontaneously, 15–20% will require some form of drainage [3]. We report the challenges we encountered during a single incision laparoscopic surgery (SILS) approach to drainage, without any specialized instruments or access platforms. To the best of our knowledge this is the second report of SILS cystogastrostomy and the first to be performed in a resource poor setting with no specialized instruments or platforms.
REPORT OF A CASE
A 33-year-old female with no medical illnesses presented to hospital complaining of epigastric pain. She was diagnosed with acute gallstone pancreatitis 2 years before but defaulted prior to cholecystectomy. On this presentation, she reported a progressively enlarging epigastric mass for approximately 6 months duration.
Abdominal ultrasound confirmed cholelithiasis and revealed the presence of a pancreatic pseudocyst containing approximately 1840 mls. A contrast-enhanced CT scan confirmed the presence of a pseudocyst closely related to the stomach (Fig. 1). Endoscopic drainage was not available at our institution. Therefore, the patient was prepared for laparoscopic internal drainage and synchronous cholecystectomy.
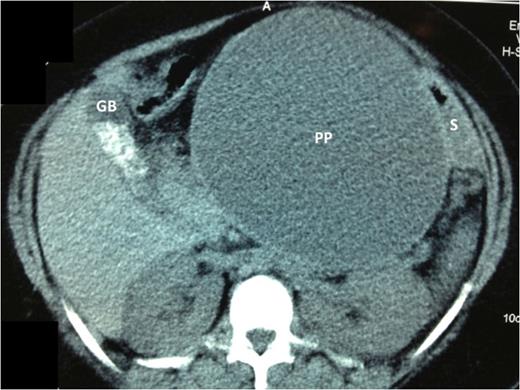
Axial cuts of a contrast enhanced CT scan of the abdomen. The large pancreatic pseudocyst (PP) is seen centrally. There is a uni-locular cavity measuring 15 × 17 cm2. The pseudocyst displaces the stomach (S) postero-laterally, but it remains closely applied to the PP wall. The gallbladder (GB) is clearly outlined on these cuts and the gallstones are seen as radio-dense opacities within its lumen.
For this procedure, a relaxing ‘S-incision’ was made at the umbilicus. Prophylactic antibiotics were administered at induction using ceftriaxone and metronidazole. The open approach was used to insert a 12 mm visual port at the inferior aspect of the incision. Two 5 mm working ports were placed beside each other at the upper end of the incision. No specialized access platforms were used. Conventional 35 mm straight laparoscopic instruments were introduced into the abdomen and used to perform the operation.
A cholecystectomy was performed first using the retrograde technique. There was significant scarring in Calot's triangle. But patient and slow dissection aided by the ‘hanging manoeuvre’ using a 2/0 polypropylene suture passed trans-cutaneously (Fig. 2), allowed us to achieve the critical view and complete the cholecystectomy.
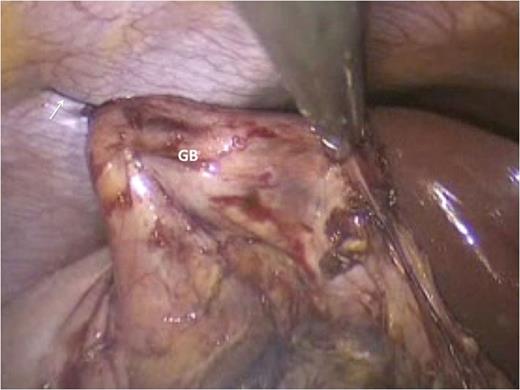
Trans-cutaneous polypropylene sutures (arrows) are used to execute the ‘hanging maneuvre’ that elevate the GB toward the antero-lateral abdominal wall. This puts Calot's triangle under stretch, thereby facilitating dissection of cystic duct and artery with electrocautery.
Attention was then turned to the pseudocyt. The stomach was seen draped over the pseudocyst, with small bowel adherent to its greater curvature. Therefore, we decided to use an anterior approach. A gastrostomy was created to open the body of the stomach (Fig. 3). This allowed the scope to enter the gastric lumen. The area of maximal pointing of the pseudocyst into the posterior wall was identified. A second gastrostomy was created at the posterior stomach wall (Fig. 4) and this was developed to enter and then decompress the pseudocyst lumen. A 60 mm Endo-GIA stapler was introduced into the tract (Fig. 5) and used to create a sturdy anastomosis between the lumina of pseudocyst and stomach (Fig. 6). The hanging manoeuvre was then used to suspend the anterior stomach wall in order to facilitate closure with the endo-GIA stapler (Fig. 7). The single ‘S’ incision in the abdomen was closed in layers. No cultures were taken since there was no clinical or biochemical evidence of infection.
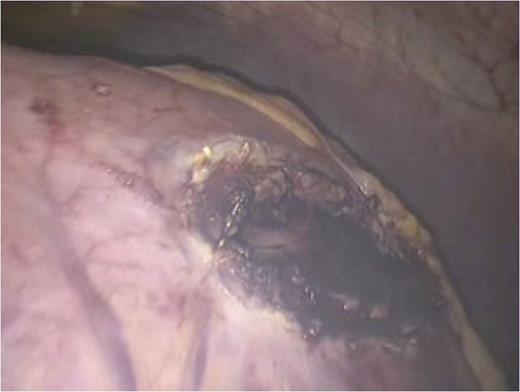
Cystogastrostomy was performed using the anterior approach. An incision was made in the anterior wall of the body of the stomach to allow entry into the gastric lumen.
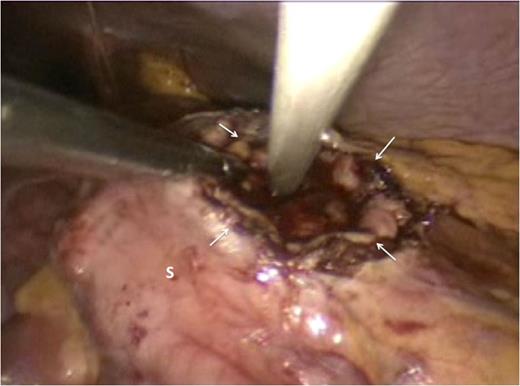
The gastric lumen was entered through the incision at the anterior stomach wall (margins indicated by white arrows). Conventional straight laparoscopic instrument can be seen developing an tunnel at the posterior stomach wall at the area where bulging from the pseudocyst is most prominent.
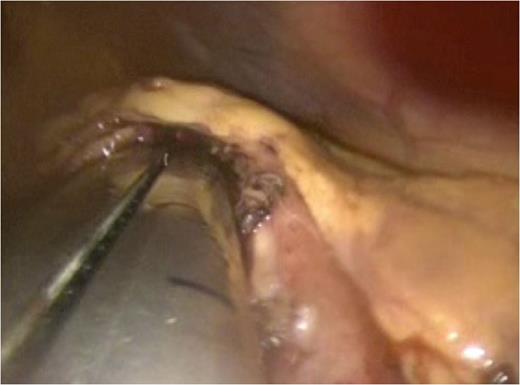
An endo-GIA articulating stapler is seen entering the anterior gastrotomy. One jaw of the stapler has been passed into the pseudocyst lumen and the other into the gastric lumen. The jaws have been approximated now in preparation for the creation of a stapled cystogastrostomy.
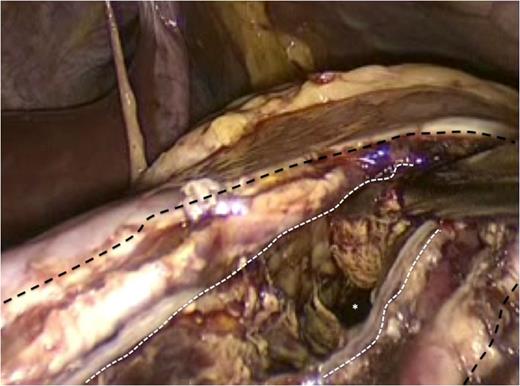
The incision at the anterior stomach wall (outlined by the broken black line) is retracted to allow access to the posterior stomach wall. An endo-GIA stapler has been fired to create a stapled anastomosis (outlined by the broken white line) between the posterior wall of the stomach and the anterior wall of the pancreatic pseudocyst. As a result the pseudocyst is opened widely to facilitate drainage of fiuid and debris into the gastric lumen.
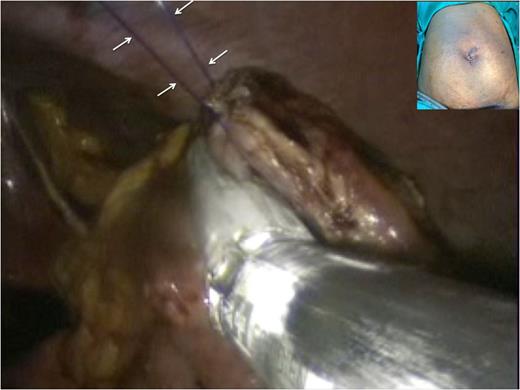
The ‘hanging manoeuvre’ is again used to suspend the margins of the anterior gastrotomy using trans-cutaneous polypropylene sutures (white arrows). This facilitates closure of the anterior gastric incision with two fires of an endo-GIA articulating stapler. The single ‘S’ incision was then closed (inset).
The patient had uneventful post-operative recovery period. She was discharged on Day 7 and had clinical resolution of the pseuodocyst. After 12 months of surveillance, there was no recurrence of the pseudocyst.
DISCUSSION
Navarra et al. reported the first SILS cholecystectomy in 1997 in Italy [4]. The first case in the Caribbean was performed over a decade after its original description [5] and since then we have accrued considerable experience with SILS cholecystectomies [6]. Although this case was technically difficult, the cholecystectomy was completed uneventfully by following our usual techniques that have already been well documented [7].
However, there are limited reports from this region for other procedures being performed using SILS. To the best of our knowledge, this is the first report of SILS cystogastrostomy from the region. A literature search also revealed that there has only been one prior published report of a SILS cystogastrostomy in the USA [8].
Lufti et al. [8] performed this first SILS cystogastrostomy in a 53-year-old woman with a large 9 × 10 cm2 pseudocyst. They used many pieces of specialized equipment to complete this procedure: EndoEYE® flexible-tip 5 mm laparoscope (Olympus), Triport access platform (Olympus), Realhand® roticulating graspers (Novare) and Endostitch® assisted suturing devices (Covidien). This allowed them to complete a hand-sewn anastomosis for the cystogastrostomy.
We practice in a low resource environment where the laparoscopic revolution is still in its infancy and procurement of specialized equipment is not prioritized [9]. Therefore, we did not have any specialized equipment at our disposal. In a way this has been beneficial because it has forced us to rely less on advanced instrumentation and to focus more on the development of laparoscopic skillsets, such as intra-corporeal suturing and the use of electrocautery for dissection.
For this case, we used conventional 35 mm straight instruments, a rigid 30o laparoscope and no access platforms. This made the procedure technically difficult with considerable instrument ‘sword-fighting’ and limited ‘tunnel-vision’ as evident in Fig. 4. However, we used previously described techniques for SILS to complete this operation. The ‘hanging manoeuvre’ [7] was quite useful in this operation and allowed us to compensate for the absence of instrument triangulation that is inherent with the performance of SILS. We have used the ‘hanging manoeuvre’ liberally in our SILS experience [7]. It is a simple, cheap and effective manoeuvre and should be in the armamentarium of surgeons performing SILS operations.
A major obstacle to the earlier adoption of SILS in the Caribbean was the cost associated with specialized SILS equipment [7]. Apart from the basic equipment, we used one Endo-GIA universal staple handle (Covidien) and two 60 mm Endo-GIA reloads. Undoubtedly, this increased the cost of this procedure, but when compared to the cost of specialized instrumentation, assisted suturing devices and the added time to complete a hand-sewn anastomosis as reported by Lufti et al. [8], we thought this was a good compromise that balanced efficacy and cost.
There were several limitations in this environment. For example, this case would have been ideal for an endoscopic cysto-gastrostomy since the pseudocyst was adherent to the posterior wall of the stomach. This required endoscopic skillsets and consumables that were not available in our setting. In any event, this patient also required a cholecystectomy that was performed concurrently at SILS.
There are no other reports of combined cystogastrostomy and cholecystectomy in the medical literature. We anticipate increasing reports of this operation since it has been demonstrated to be feasible. We advocate performing the cholecystectomy first step for two reasons. Firstly, it will prevent any bleeding from the cystogastrostomy to obscure the field when dissecting Calot's triangle. More importantly, it will prevent inadvertent traction on the cystogastrostomy anastomosis while performing cholecystectomy.
CONCLUSIONS
Advanced procedures such as cystogastrostomy can be performed using the SILS approach, even in resource poor settings with basic equipment. In an attempt to balance the costs associated with SILS, surgeons should consider this as a feasible option. These are complex procedures and should be performed by laparoscopic surgeons with experience in SILS to minimize morbidity with these technically challenging operations.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest to disclose.
FUNDING
There have been no sources of funding for this manuscript.



