-
PDF
- Split View
-
Views
-
Cite
Cite
Edward J. Woo, Aaron D. Baugh, Karen Ching, Synchronous presentation of invasive ductal carcinoma and mantle cell lymphoma: a diagnostic challenge in menopausal patients, Journal of Surgical Case Reports, Volume 2016, Issue 1, January 2016, rjv153, https://doi.org/10.1093/jscr/rjv153
Close - Share Icon Share
Abstract
Synchronous presentation of breast carcinoma and non-Hodgkin lymphoma (NHL) is a rare occurrence (Bradford PT, Freedman DM, Goldstein AM, Tucker MA. Increased risk of second primary cancers after a diagnosis of melanoma. Arch Dermatol 2010;146:265–72; Dutta Roy S, Stafford JA, Scally J, Selvachandran SN. A rare case of breast carcinoma co-existing with axillary mantle cell lymphoma. World J Surg Oncol 2003;1:27; Suresh Attili VS, Dadhich HK, Rao CR, Bapsy PP, Batra U, Anupama G et al. A case of breast cancer coexisting with B-cell follicular lymphoma. Austral Asian J Cancer 2007;6:155–6). In particular, only two reported cases on synchronous presentation of invasive ductal carcinoma (IDC) and mantle cell lymphoma (MCL) exist in the English literature. Owing to the rarity, there is a lack of consensus about underlying mechanism as well as optimal treatment strategy, and diagnosing both malignancies together without a delay remains a complex clinical challenge. We report a case of synchronous presentation of IDC and MCL in a 67-year-old female patient whose MCL diagnosis was delayed due to a misinterpretation of her B symptoms as postmenopausal, with a review of the literature on concurrently occurring breast carcinoma and NHL.
INTRODUCTION
Cancer survivors are at increased risk for new primary malignancies [1]. However, a concurrent presentation of multiple, primary neoplasms is a rare occurrence [2, 3]. At present, it is unclear whether or not they arise through common underlying mechanisms, one triggering the other, or whether each disease process is entirely independent of another. Likewise, there is a lack of consensus about optimal treatment strategy.
Of the 37 known cases of synchronous, non-Hodgkin lymphoma (NHL) and breast cancer, most exist only as case reports or small case series with limited follow-ups. Here, we present only the third reported case of synchronous, mantle cell lymphoma (MCL), a rare form of malignancy that accounts for 3–6% of NHL [4] and invasive ductal carcinoma (IDC).
CASE REPORT
A 67-year-old female presented with a biopsy-proven and palpable IDC of the left breast. The patient had had two previous breast biopsies, both benign, over a decade ago. Her last mammogram from 2 years ago was without suspicious findings, and the patient had appreciated no lesion in the last 6 months of self-breast examinations. Her family history was significant for breast cancer in her mother, diagnosed at age 41. Her past medical history was otherwise notable for hypothyroidism, psoriatic arthritis, and an excised basal cell carcinoma of the chest wall. Mammography showed a 2.4 cm × 1.4 cm × 1.8 cm lobulated mass with indistinct margins in the left breast (Fig. 1).
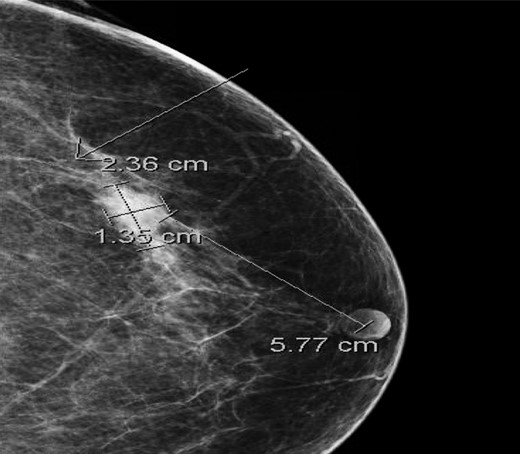
Cranio-caudal view of mammogram showing a 2.4 cm × 1.4 cm × 1.8 cm lobulated mass with an indistinct margin in the left breast at 1 o'clock, 5.8 cm from the nipple.
At presentation, she did not complain of any fevers, night sweats or unintentional weight loss. During her physical examination, there was a suspicious palpable lymph node in the left axilla. However, preoperative fine-needle aspiration biopsy (FNAB) of this node showed no evidence of metastatic breast cancer. The patient proceeded to surgery for a lumpectomy with a sentinel lymph node biopsy. Final pathology revealed a 1.6-cm focus of T1cN0M0 ER-/PR-IDC with the 3+ presence of Her-2 on immunohistochemistry (Fig. 2), and some surrounding ductal carcinoma in situ. The sampled sentinel lymph nodes were negative for metastasis, but MCL was found in 7 out of 7 of the nodes (Fig. 3).
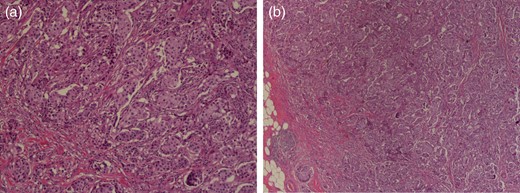
Histologic images of the IDC. Magnification ×100. (a) Hematoxylin and eosin (H&E) staining photomicrograph of left breast cancer showing proliferative growth of malignant ductal epithelial cells and (b) invasion under basement membrane.
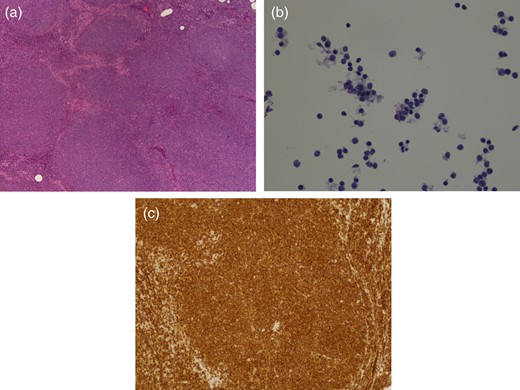
Histologic images of MCL. Magnification ×100. (a) H&E staining showing effaced nodal architecture due to closely packed neoplastic growth of mantle zone B-cells of lymphoid follicles, (b) singly scattered epitheliod histocytes making a starry-sky appearance at a lower magnification and (c) cyclin D1+ immunostaining.
Subsequently, staging work-ups of the incidental tumors revealed synchronous stage IVB MCL. In subsequent interviews, the patient admitted several months of night sweats prior to her breast cancer diagnosis. A PET/CT scan demonstrated 18F-fluorodeoxyglucose-avid lymphadenopathy of the neck, axilla and mediastinum that were consistent with the patient's MCL (Fig. 4). She then underwent a colonoscopy and upper endoscopy without evidence of gastrointestinal involvement. Her case was discussed in a multidisciplinary breast oncology conference. The patient ultimately underwent three cycles of R-CHOPP chemotherapy and three cycles of R-DHAP with Trastuzumab. She tolerated it well without major complications. She is currently planned for R-BEAM consolidation chemotherapy and subsequent autologous bone marrow transplantation, followed by adjuvant breast irradiation.
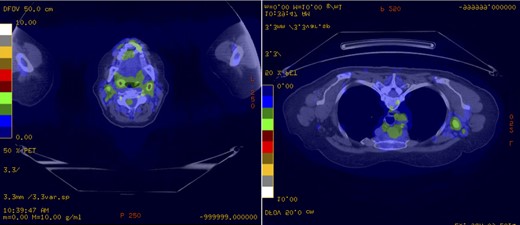
PET demonstrating 18F-fluorodeoxyglucose-avid lymphadenopathy of the neck, axilla and mediastinum consistent with the patient's MCL. (a) The largest lymph nodes in the left neck level II measured 16 × 13 mm with a maximal SUV of 4.8. (b) The largest left axillary lymph node measured 26 × 18 mm with an associated maximal SUV of 3.7.
DISCUSSION
The present case begs for discussion on several issues regarding the synchronous presentation of MCL and IDC—cause of a delay in diagnosing second primary malignancy, implication of such a delay in overall treatment strategy and prognosis and correlation between two pathological processes in their synchronous presentation.
In our review of the 37 reported cases of concurrent breast cancer and NHL of all types, it was noted that 88.9% (32/36) failed to detect the second malignancy until the initiation of definitive treatments for the first. Considering how breast cancer was first detected prior to NHL in 89.5% (34/38) of the cases with the majority of patients being peri- or postmenopausal (Table 1), the missed diagnoses could potentially be attributed to perimenopausal signs masking B symptoms of NHL. This was the case for our patient. Hot flashes, weight loss, fever and night sweats are frequently featured in both menopausal and neoplastic processes. Also, lymphadenopathy, especially when confined to the axillae, can be easily misattributed to known breast cancer. Even clinically detected lymph nodes may not produce reliable diagnostics. Two cases, including our own, reported negative FNAB in patients with advanced NHL. At present, it is unclear whether this represents a characteristic of synchronous breast cancer and NHL, or if this is a normal variance, given that the sensitivity of axillary FNA is only estimated at 63% [5].
| Case # . | . | BC . | NHL/HL . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/S . | Side . | Histol . | Stage . | Ax FNA . | LAN . | Histol . | Biopsy . | Stage . | Rec/Met . | Ref. . | Year . | |
| BC first (MCL) | ||||||||||||
| 1 | 67/F | L | IDC | IA | − | + | MCL | AxLN | IV B | – | 2014 | |
| 2 | 67/F | L | IDC | IA | + | + | MCL | AxLN | I A | 2003 | ||
| 3 | 63/F | L | IDC | IA | + | + | MCL | AxLN | III A | 2006 | ||
| BC first (other NHLs) | ||||||||||||
| 4 | 51/F | L | IDC | III | − | − | DLBCL | AxLN | Met | 2014 | ||
| 5 | 51/F | L | IDC | III | − | − | DLBCL | AxLN | – | 2014 | ||
| 6 | 51/F | L | IDC | IA | DLBCL | AxLN | IVB | – | 2014 | |||
| 7 | 47/F | R | IDC | DLBCL | AxLN | IB | – | |||||
| 8 | 49/F | R | DCIS | 0 | + | FL | AxLN | IIIA | 2014 | |||
| 9 | 40/F | R | IDC | IV | − | + | FL | AxLN | IVA | Met | 2014 | |
| 10 | 82/F | L | ILC | IV | + | (+) | DLBCL | AxLN | IA | Met | 2014 | |
| 11 | 78/F | L | IDC | + | + | DLBCL | AxLN | IIIA | Rec | 2011 | ||
| 12 | 74/F | ILC | FL | I | – | 2011 | ||||||
| 13 | 74/F | IDC | IIB | − | + | CLL | AxLN | 0 | Met | |||
| 14 | 54/F | IDC | IIA | − | + | CLL | AxLN | – | ||||
| 15 | 47/F | IDC | + | NOS | 2011 | |||||||
| 16 | 87/F | IDC | IIA | − | CLL | 0 | 2011 | |||||
| 17 | 87/F | IDC | IIIA | CLL | AxLN | 2010 | ||||||
| 18 | 69/F | IDC | IIB | CLL | AxLN | |||||||
| 19 | 86/F | IDC | IIB | CLL | AxLN | |||||||
| 20 | 83/F | IDC | IIIA | CLL | AxLN | |||||||
| 21 | 52/F | IDC | IIB | − | FL | AxLN | IA | Met | 2010 | |||
| 22 | 56/F | ILC | IA | MZBL | AxLN | IV | – | 2008 | ||||
| 23 | 57/F | R | IDC | I | − | MZBL | AxLN | IIA | 2008 | |||
| 24 | 50/F | L | IDC | IA | − | FL | IIIA | 2006 | ||||
| 25 | 58/F | L | DCIS | − | FL | IA | ||||||
| 26 | 53/F | IDC | IIA | − | MALT | 2006 | ||||||
| 27 | 61/F | IDC | IA | − | FL | AxLN | IIIA | 2005 | ||||
| 28 | 79/F | IDC | IIA | MALT | IVA | 2004 | ||||||
| 29 | 62/F | BL | IDC | IA | − | DLBCL | AxLN | I | – | 2002 | ||
| 30 | 67/F | IDC | IIB | − | FL | AxLN | IV | – | 1999 | |||
| 31 | 77/F | BL | SC | + | AxLN | IIIA | 1998 | |||||
| 32 | 87/F | ILC | IA | DLBCL | AxLN | IV | – | 1998 | ||||
| 33 | 66/F | R | IDC | IA | + | FL | AxLN | 1990 | ||||
| 34 | 77/F | IDC | IA | − | FL | AxLN | ||||||
| NHL first | ||||||||||||
| 35 | 75/F | IDC | IA | DLBCL | AxLN | 2014 | ||||||
| 36 | 64/F | R | IDC | IIA | + | DLBCL | AxLN | IIIB | 2012 | |||
| 37 | 52/F | L | IDC | IIA | + | DLBCL | AxLN | 2011 | ||||
| 38 | 60/F | IDC | + | NOS | 2009 | |||||||
| Case # . | . | BC . | NHL/HL . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/S . | Side . | Histol . | Stage . | Ax FNA . | LAN . | Histol . | Biopsy . | Stage . | Rec/Met . | Ref. . | Year . | |
| BC first (MCL) | ||||||||||||
| 1 | 67/F | L | IDC | IA | − | + | MCL | AxLN | IV B | – | 2014 | |
| 2 | 67/F | L | IDC | IA | + | + | MCL | AxLN | I A | 2003 | ||
| 3 | 63/F | L | IDC | IA | + | + | MCL | AxLN | III A | 2006 | ||
| BC first (other NHLs) | ||||||||||||
| 4 | 51/F | L | IDC | III | − | − | DLBCL | AxLN | Met | 2014 | ||
| 5 | 51/F | L | IDC | III | − | − | DLBCL | AxLN | – | 2014 | ||
| 6 | 51/F | L | IDC | IA | DLBCL | AxLN | IVB | – | 2014 | |||
| 7 | 47/F | R | IDC | DLBCL | AxLN | IB | – | |||||
| 8 | 49/F | R | DCIS | 0 | + | FL | AxLN | IIIA | 2014 | |||
| 9 | 40/F | R | IDC | IV | − | + | FL | AxLN | IVA | Met | 2014 | |
| 10 | 82/F | L | ILC | IV | + | (+) | DLBCL | AxLN | IA | Met | 2014 | |
| 11 | 78/F | L | IDC | + | + | DLBCL | AxLN | IIIA | Rec | 2011 | ||
| 12 | 74/F | ILC | FL | I | – | 2011 | ||||||
| 13 | 74/F | IDC | IIB | − | + | CLL | AxLN | 0 | Met | |||
| 14 | 54/F | IDC | IIA | − | + | CLL | AxLN | – | ||||
| 15 | 47/F | IDC | + | NOS | 2011 | |||||||
| 16 | 87/F | IDC | IIA | − | CLL | 0 | 2011 | |||||
| 17 | 87/F | IDC | IIIA | CLL | AxLN | 2010 | ||||||
| 18 | 69/F | IDC | IIB | CLL | AxLN | |||||||
| 19 | 86/F | IDC | IIB | CLL | AxLN | |||||||
| 20 | 83/F | IDC | IIIA | CLL | AxLN | |||||||
| 21 | 52/F | IDC | IIB | − | FL | AxLN | IA | Met | 2010 | |||
| 22 | 56/F | ILC | IA | MZBL | AxLN | IV | – | 2008 | ||||
| 23 | 57/F | R | IDC | I | − | MZBL | AxLN | IIA | 2008 | |||
| 24 | 50/F | L | IDC | IA | − | FL | IIIA | 2006 | ||||
| 25 | 58/F | L | DCIS | − | FL | IA | ||||||
| 26 | 53/F | IDC | IIA | − | MALT | 2006 | ||||||
| 27 | 61/F | IDC | IA | − | FL | AxLN | IIIA | 2005 | ||||
| 28 | 79/F | IDC | IIA | MALT | IVA | 2004 | ||||||
| 29 | 62/F | BL | IDC | IA | − | DLBCL | AxLN | I | – | 2002 | ||
| 30 | 67/F | IDC | IIB | − | FL | AxLN | IV | – | 1999 | |||
| 31 | 77/F | BL | SC | + | AxLN | IIIA | 1998 | |||||
| 32 | 87/F | ILC | IA | DLBCL | AxLN | IV | – | 1998 | ||||
| 33 | 66/F | R | IDC | IA | + | FL | AxLN | 1990 | ||||
| 34 | 77/F | IDC | IA | − | FL | AxLN | ||||||
| NHL first | ||||||||||||
| 35 | 75/F | IDC | IA | DLBCL | AxLN | 2014 | ||||||
| 36 | 64/F | R | IDC | IIA | + | DLBCL | AxLN | IIIB | 2012 | |||
| 37 | 52/F | L | IDC | IIA | + | DLBCL | AxLN | 2011 | ||||
| 38 | 60/F | IDC | + | NOS | 2009 | |||||||
BC, breast cancer; NHL, Non-Hodgkin lymphoma; MCL, mantle cell lymphoma; FL, follicular lymphoma; MZBL, marginal zone B-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; CLL, chronic lymphoid leukemia; NOS, not otherwise specified; MALT, mucosa-associated lymphoid tissue lymphoma; R, right; L, left; BL, bilateral; Br, breast; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; SC, spindle cell carcinoma; T, tumor; N, node; M, metastasis; LN, lymph node; Ax, axillary; Met, metastasis; Rec, recurrence; N, no recurrence.
| Case # . | . | BC . | NHL/HL . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/S . | Side . | Histol . | Stage . | Ax FNA . | LAN . | Histol . | Biopsy . | Stage . | Rec/Met . | Ref. . | Year . | |
| BC first (MCL) | ||||||||||||
| 1 | 67/F | L | IDC | IA | − | + | MCL | AxLN | IV B | – | 2014 | |
| 2 | 67/F | L | IDC | IA | + | + | MCL | AxLN | I A | 2003 | ||
| 3 | 63/F | L | IDC | IA | + | + | MCL | AxLN | III A | 2006 | ||
| BC first (other NHLs) | ||||||||||||
| 4 | 51/F | L | IDC | III | − | − | DLBCL | AxLN | Met | 2014 | ||
| 5 | 51/F | L | IDC | III | − | − | DLBCL | AxLN | – | 2014 | ||
| 6 | 51/F | L | IDC | IA | DLBCL | AxLN | IVB | – | 2014 | |||
| 7 | 47/F | R | IDC | DLBCL | AxLN | IB | – | |||||
| 8 | 49/F | R | DCIS | 0 | + | FL | AxLN | IIIA | 2014 | |||
| 9 | 40/F | R | IDC | IV | − | + | FL | AxLN | IVA | Met | 2014 | |
| 10 | 82/F | L | ILC | IV | + | (+) | DLBCL | AxLN | IA | Met | 2014 | |
| 11 | 78/F | L | IDC | + | + | DLBCL | AxLN | IIIA | Rec | 2011 | ||
| 12 | 74/F | ILC | FL | I | – | 2011 | ||||||
| 13 | 74/F | IDC | IIB | − | + | CLL | AxLN | 0 | Met | |||
| 14 | 54/F | IDC | IIA | − | + | CLL | AxLN | – | ||||
| 15 | 47/F | IDC | + | NOS | 2011 | |||||||
| 16 | 87/F | IDC | IIA | − | CLL | 0 | 2011 | |||||
| 17 | 87/F | IDC | IIIA | CLL | AxLN | 2010 | ||||||
| 18 | 69/F | IDC | IIB | CLL | AxLN | |||||||
| 19 | 86/F | IDC | IIB | CLL | AxLN | |||||||
| 20 | 83/F | IDC | IIIA | CLL | AxLN | |||||||
| 21 | 52/F | IDC | IIB | − | FL | AxLN | IA | Met | 2010 | |||
| 22 | 56/F | ILC | IA | MZBL | AxLN | IV | – | 2008 | ||||
| 23 | 57/F | R | IDC | I | − | MZBL | AxLN | IIA | 2008 | |||
| 24 | 50/F | L | IDC | IA | − | FL | IIIA | 2006 | ||||
| 25 | 58/F | L | DCIS | − | FL | IA | ||||||
| 26 | 53/F | IDC | IIA | − | MALT | 2006 | ||||||
| 27 | 61/F | IDC | IA | − | FL | AxLN | IIIA | 2005 | ||||
| 28 | 79/F | IDC | IIA | MALT | IVA | 2004 | ||||||
| 29 | 62/F | BL | IDC | IA | − | DLBCL | AxLN | I | – | 2002 | ||
| 30 | 67/F | IDC | IIB | − | FL | AxLN | IV | – | 1999 | |||
| 31 | 77/F | BL | SC | + | AxLN | IIIA | 1998 | |||||
| 32 | 87/F | ILC | IA | DLBCL | AxLN | IV | – | 1998 | ||||
| 33 | 66/F | R | IDC | IA | + | FL | AxLN | 1990 | ||||
| 34 | 77/F | IDC | IA | − | FL | AxLN | ||||||
| NHL first | ||||||||||||
| 35 | 75/F | IDC | IA | DLBCL | AxLN | 2014 | ||||||
| 36 | 64/F | R | IDC | IIA | + | DLBCL | AxLN | IIIB | 2012 | |||
| 37 | 52/F | L | IDC | IIA | + | DLBCL | AxLN | 2011 | ||||
| 38 | 60/F | IDC | + | NOS | 2009 | |||||||
| Case # . | . | BC . | NHL/HL . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/S . | Side . | Histol . | Stage . | Ax FNA . | LAN . | Histol . | Biopsy . | Stage . | Rec/Met . | Ref. . | Year . | |
| BC first (MCL) | ||||||||||||
| 1 | 67/F | L | IDC | IA | − | + | MCL | AxLN | IV B | – | 2014 | |
| 2 | 67/F | L | IDC | IA | + | + | MCL | AxLN | I A | 2003 | ||
| 3 | 63/F | L | IDC | IA | + | + | MCL | AxLN | III A | 2006 | ||
| BC first (other NHLs) | ||||||||||||
| 4 | 51/F | L | IDC | III | − | − | DLBCL | AxLN | Met | 2014 | ||
| 5 | 51/F | L | IDC | III | − | − | DLBCL | AxLN | – | 2014 | ||
| 6 | 51/F | L | IDC | IA | DLBCL | AxLN | IVB | – | 2014 | |||
| 7 | 47/F | R | IDC | DLBCL | AxLN | IB | – | |||||
| 8 | 49/F | R | DCIS | 0 | + | FL | AxLN | IIIA | 2014 | |||
| 9 | 40/F | R | IDC | IV | − | + | FL | AxLN | IVA | Met | 2014 | |
| 10 | 82/F | L | ILC | IV | + | (+) | DLBCL | AxLN | IA | Met | 2014 | |
| 11 | 78/F | L | IDC | + | + | DLBCL | AxLN | IIIA | Rec | 2011 | ||
| 12 | 74/F | ILC | FL | I | – | 2011 | ||||||
| 13 | 74/F | IDC | IIB | − | + | CLL | AxLN | 0 | Met | |||
| 14 | 54/F | IDC | IIA | − | + | CLL | AxLN | – | ||||
| 15 | 47/F | IDC | + | NOS | 2011 | |||||||
| 16 | 87/F | IDC | IIA | − | CLL | 0 | 2011 | |||||
| 17 | 87/F | IDC | IIIA | CLL | AxLN | 2010 | ||||||
| 18 | 69/F | IDC | IIB | CLL | AxLN | |||||||
| 19 | 86/F | IDC | IIB | CLL | AxLN | |||||||
| 20 | 83/F | IDC | IIIA | CLL | AxLN | |||||||
| 21 | 52/F | IDC | IIB | − | FL | AxLN | IA | Met | 2010 | |||
| 22 | 56/F | ILC | IA | MZBL | AxLN | IV | – | 2008 | ||||
| 23 | 57/F | R | IDC | I | − | MZBL | AxLN | IIA | 2008 | |||
| 24 | 50/F | L | IDC | IA | − | FL | IIIA | 2006 | ||||
| 25 | 58/F | L | DCIS | − | FL | IA | ||||||
| 26 | 53/F | IDC | IIA | − | MALT | 2006 | ||||||
| 27 | 61/F | IDC | IA | − | FL | AxLN | IIIA | 2005 | ||||
| 28 | 79/F | IDC | IIA | MALT | IVA | 2004 | ||||||
| 29 | 62/F | BL | IDC | IA | − | DLBCL | AxLN | I | – | 2002 | ||
| 30 | 67/F | IDC | IIB | − | FL | AxLN | IV | – | 1999 | |||
| 31 | 77/F | BL | SC | + | AxLN | IIIA | 1998 | |||||
| 32 | 87/F | ILC | IA | DLBCL | AxLN | IV | – | 1998 | ||||
| 33 | 66/F | R | IDC | IA | + | FL | AxLN | 1990 | ||||
| 34 | 77/F | IDC | IA | − | FL | AxLN | ||||||
| NHL first | ||||||||||||
| 35 | 75/F | IDC | IA | DLBCL | AxLN | 2014 | ||||||
| 36 | 64/F | R | IDC | IIA | + | DLBCL | AxLN | IIIB | 2012 | |||
| 37 | 52/F | L | IDC | IIA | + | DLBCL | AxLN | 2011 | ||||
| 38 | 60/F | IDC | + | NOS | 2009 | |||||||
BC, breast cancer; NHL, Non-Hodgkin lymphoma; MCL, mantle cell lymphoma; FL, follicular lymphoma; MZBL, marginal zone B-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; CLL, chronic lymphoid leukemia; NOS, not otherwise specified; MALT, mucosa-associated lymphoid tissue lymphoma; R, right; L, left; BL, bilateral; Br, breast; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; SC, spindle cell carcinoma; T, tumor; N, node; M, metastasis; LN, lymph node; Ax, axillary; Met, metastasis; Rec, recurrence; N, no recurrence.
During the review, it was impractical to draw any conclusion on how and to what extent discovering breast cancer, prior to NHL or vice versa, alters overall clinical outcome due to numerous, confounding variables such as cancer subtype and stage. The fact that the gap of 52 days, between our patient's IDC diagnosis and the initiation of chemotherapy of MCL, was well within the median and recommended time frame of 11 weeks and 120 days [6, 7] makes it even harder to assess the impact of diagnostic delay. Currently, pathological stage and type of NHL often dictate treatment strategies for patients with a double presentation of breast cancer and NHL [8]. This involves the use of intensive multiagent, chemotherapy induction followed by a consolidation and maintenance phase [8]. However, MCL, due to its rarity, high recurrences and a median overall survival of only 4–5 years [9], is still considered incurable [10], and the choice of treatment in the presence of another primary malignancy remains a complex problem.
The same argument also challenged our attempt to observe any relationship between two malignancies. In the three reported cases of synchronous MCL and IDC, including the present one, the stage of IDC was unanimously IA, whereas the stage of MCL greatly differed from one another, ranging from IA to IVB (Table 2). Consistent with this, the majority of the reported cases demonstrated a wide disparity between the stages of the two diseases. One's growth may be inhibited by the same factors fueling the progression of the second, or may be completely independent of one another.
| Characteristics . | Patient . | ||
|---|---|---|---|
| 1 . | 2 . | Present case . | |
| Age/gender | 67/F | 63/F | 67/F |
| First noted | BC | BC | BC |
| Palpable breast mass | − | + | + |
| Lymphedema | − | + | + |
| Ax FNA result | − | ||
| BC | |||
| Side | Left | Left | Left |
| Histology | IDC | IDC | IDC |
| Grade | 2 | 1 | 3 |
| T | 1b | 1c | |
| N | 0 | 0 | |
| M | 0 | 0 | |
| Stage | IA | IA | IA |
| ER | + | + | − |
| PgR | − | + | − |
| HER2 | + | ||
| Surgery | Lump + SLN | Lump + SLN | Lump + SLN |
| Adjuvant Tx | RT + T | RT + T | R-CHOPP × 3 + T |
| MCL | |||
| B Sx | N | N | Y |
| Biopsy site | AxLN | AxLN | AxLN |
| Grade | |||
| Stage | IA | IIIA | IVB |
| CD5 | + | + | + |
| CD10 | + | − | |
| CD20 | + | + | + |
| CD23 | − | − | |
| BCL2 | + | ||
| BCL6 | + | − | |
| Cyclin D1 | + | + | + |
| Therapy | Observation | ABCM × 4 | R-DHAP × 3 |
| Reference | [5] | [10] | |
| Year | 2003 | 2006 | 2014 |
| Characteristics . | Patient . | ||
|---|---|---|---|
| 1 . | 2 . | Present case . | |
| Age/gender | 67/F | 63/F | 67/F |
| First noted | BC | BC | BC |
| Palpable breast mass | − | + | + |
| Lymphedema | − | + | + |
| Ax FNA result | − | ||
| BC | |||
| Side | Left | Left | Left |
| Histology | IDC | IDC | IDC |
| Grade | 2 | 1 | 3 |
| T | 1b | 1c | |
| N | 0 | 0 | |
| M | 0 | 0 | |
| Stage | IA | IA | IA |
| ER | + | + | − |
| PgR | − | + | − |
| HER2 | + | ||
| Surgery | Lump + SLN | Lump + SLN | Lump + SLN |
| Adjuvant Tx | RT + T | RT + T | R-CHOPP × 3 + T |
| MCL | |||
| B Sx | N | N | Y |
| Biopsy site | AxLN | AxLN | AxLN |
| Grade | |||
| Stage | IA | IIIA | IVB |
| CD5 | + | + | + |
| CD10 | + | − | |
| CD20 | + | + | + |
| CD23 | − | − | |
| BCL2 | + | ||
| BCL6 | + | − | |
| Cyclin D1 | + | + | + |
| Therapy | Observation | ABCM × 4 | R-DHAP × 3 |
| Reference | [5] | [10] | |
| Year | 2003 | 2006 | 2014 |
BC, breast cancer; MCL, mantle cell lymphoma; metachro, metachronous; synchro, synchronous; R, right; L, left; Br, breast; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; T, tumor; N, node; M, metastasis; MX, mastectomy; RT, radiotherapy; CD, cluster of differentiation; LN, lymph node; Ax, axillary; RT, radiation therapy; R-CHOP, rituximab + cyclophosphamide + hydroxydaunorubicin + oncovin + prednisone or prednisolone; R-DHAP, rituximab + dexamethasone + high-dose ara-C-cytarabine + platinol (cisplatin); T, tamoxifen; ABCM, doxorubicin (Adriamycin) + carmustine (BCNU) + cyclophosphamide + melphalan.
| Characteristics . | Patient . | ||
|---|---|---|---|
| 1 . | 2 . | Present case . | |
| Age/gender | 67/F | 63/F | 67/F |
| First noted | BC | BC | BC |
| Palpable breast mass | − | + | + |
| Lymphedema | − | + | + |
| Ax FNA result | − | ||
| BC | |||
| Side | Left | Left | Left |
| Histology | IDC | IDC | IDC |
| Grade | 2 | 1 | 3 |
| T | 1b | 1c | |
| N | 0 | 0 | |
| M | 0 | 0 | |
| Stage | IA | IA | IA |
| ER | + | + | − |
| PgR | − | + | − |
| HER2 | + | ||
| Surgery | Lump + SLN | Lump + SLN | Lump + SLN |
| Adjuvant Tx | RT + T | RT + T | R-CHOPP × 3 + T |
| MCL | |||
| B Sx | N | N | Y |
| Biopsy site | AxLN | AxLN | AxLN |
| Grade | |||
| Stage | IA | IIIA | IVB |
| CD5 | + | + | + |
| CD10 | + | − | |
| CD20 | + | + | + |
| CD23 | − | − | |
| BCL2 | + | ||
| BCL6 | + | − | |
| Cyclin D1 | + | + | + |
| Therapy | Observation | ABCM × 4 | R-DHAP × 3 |
| Reference | [5] | [10] | |
| Year | 2003 | 2006 | 2014 |
| Characteristics . | Patient . | ||
|---|---|---|---|
| 1 . | 2 . | Present case . | |
| Age/gender | 67/F | 63/F | 67/F |
| First noted | BC | BC | BC |
| Palpable breast mass | − | + | + |
| Lymphedema | − | + | + |
| Ax FNA result | − | ||
| BC | |||
| Side | Left | Left | Left |
| Histology | IDC | IDC | IDC |
| Grade | 2 | 1 | 3 |
| T | 1b | 1c | |
| N | 0 | 0 | |
| M | 0 | 0 | |
| Stage | IA | IA | IA |
| ER | + | + | − |
| PgR | − | + | − |
| HER2 | + | ||
| Surgery | Lump + SLN | Lump + SLN | Lump + SLN |
| Adjuvant Tx | RT + T | RT + T | R-CHOPP × 3 + T |
| MCL | |||
| B Sx | N | N | Y |
| Biopsy site | AxLN | AxLN | AxLN |
| Grade | |||
| Stage | IA | IIIA | IVB |
| CD5 | + | + | + |
| CD10 | + | − | |
| CD20 | + | + | + |
| CD23 | − | − | |
| BCL2 | + | ||
| BCL6 | + | − | |
| Cyclin D1 | + | + | + |
| Therapy | Observation | ABCM × 4 | R-DHAP × 3 |
| Reference | [5] | [10] | |
| Year | 2003 | 2006 | 2014 |
BC, breast cancer; MCL, mantle cell lymphoma; metachro, metachronous; synchro, synchronous; R, right; L, left; Br, breast; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; T, tumor; N, node; M, metastasis; MX, mastectomy; RT, radiotherapy; CD, cluster of differentiation; LN, lymph node; Ax, axillary; RT, radiation therapy; R-CHOP, rituximab + cyclophosphamide + hydroxydaunorubicin + oncovin + prednisone or prednisolone; R-DHAP, rituximab + dexamethasone + high-dose ara-C-cytarabine + platinol (cisplatin); T, tamoxifen; ABCM, doxorubicin (Adriamycin) + carmustine (BCNU) + cyclophosphamide + melphalan.
Synchronous presentation of breast cancer and NHL is a rare, complex clinical challenge. A better long-term follow-up is needed to develop evidence-based protocols that adequately address the special issues that arise in the care of these patients. In particular, we emphasize the importance of a comprehensive review of systems and a more attentive physical examination of patients for improved detection of these synchronous malignancies.
CONFLICT OF INTEREST STATEMENT
None declared.



