-
PDF
- Split View
-
Views
-
Cite
Cite
Hideyuki Maeda, Masato Kanzaki, Tamami Isaka, Takamasa Onuki, Video-assisted thoracoscopic enucleation after congenital cardiac surgery, Journal of Surgical Case Reports, Volume 2015, Issue 9, September 2015, rjv123, https://doi.org/10.1093/jscr/rjv123
Close - Share Icon Share
Abstract
A 26-year-old man underwent arterial switch surgery for transposition of the great arteries in infancy. During a routine evaluation, a nodule was detected in the left lower lobe on chest computed tomography. The tumor had enlarged at follow-up and he underwent surgical resection. Because of past cardiac surgery, the pericardium was defective; therefore, the heart was exposed to the pleural cavity and severe adhesions surrounding the left lung. We had to encircle the left main pulmonary artery to perform enucleation safely. The tumor was diagnosed as a pulmonary sclerosing hemangioma using permanent pathology results.
INTRODUCTION
The long-term outcomes of cardiac surgery have recently been improved, and general thoracic surgeons often perform lung surgery after cardiac surgery. It is often difficult to dissect adhesions in the pleural cavity altered during prior cardiac surgery. We report a case of video-assisted thoracoscopic enucleation in an adult patient with congenital heart disease surgically repaired during infancy.
CASE REPORT
A 26-year-old man underwent arterial switch surgery for transposition of the great arteries in infancy. During a routine evaluation, a nodule was detected in the lower lobe of the left lung on chest computed tomography (CT). At the follow-up examination, the size of the tumor had increased 5 mm each year and he was referred for surgical treatment. Chest CT showed a 40-mm tumor with a smooth margin and a heterogenous inner structure in the left S8 pulmonary segment, close to the interlobar pulmonary artery (PA; Fig. 1). Tumor markers, such as carcinoembryonic antigen, cytokeratine 19 fragment and carbohydrate antigen 19-9, were within the normal range. Positron emission tomography (PET) showed abnormal F-18 fludeoxyglucose (FDG) uptake in the tumor shadow, and the maximum standardized uptake value was 4.66. There was no abnormal FDG uptake in the hilar or mediastinal lymph nodes. We planned to select the operative procedure using intraoperative frozen section examination, because it was difficult to diagnose whether the tumor was benign or malignant.
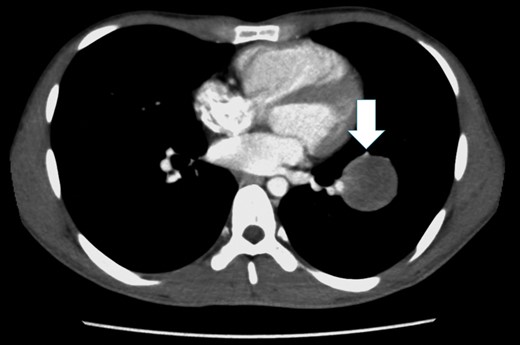
Chest CT shows a tumor with a smooth margin in the left S8 pulmonary segment, close to the interlobar PA, and the lingular segmental artery is compressed by the tumor (white arrow).
The patient was anesthetized with general anesthesia, intubated with a double-lumen endotracheal tube intubation and placed in a left lateral decubitus position. A 5-cm utility minithoracotomy was placed in the fifth intercostal space (ICS), and two ports were placed in the eighth ICS at the middle and posterior axillary lines. All procedures were performed under thoracoscopic view. The pericardium was defective, and the epicardium was exposed to the pleural cavity. There was direct adhesion between the exposed heart and lung; therefore, dissection of the adhesion was performed with great caution (Fig. 2). The tumor was predominantly located in the lower lobe, over the left major fissure, and spreads to the left upper lobe (Fig. 3). We decided to perform tumor enucleation, because the tumor was diagnosed as inflammatory or fibrotic upon intraoperative frozen section examination. Because of a severe adhesion surrounding the left pulmonary hilum, it was considered too difficult to tape the left main PA immediately after bleeding from the interlobar PA. First, the left upper pulmonary vein was encircled and pulled caudally (Fig. 4). Then, the left main PA was encircled prior to enucleation to prepare for the emergent bleeding. This procedure was performed through an additional anterolateral minithoracotomy (3 cm) in the third ICS. An adhesion surrounding the left PA was especially severe and very difficult to dissect. The vascular tape encircling the left main PA was extracted to outside of the pleural cavity through the additional minithoracotomy, and we prepared for clamping when needed. Then, tumor enucleation was performed. An adhesion between the tumor and the interlobar PA was safely dissected. We could control an air leakage by using a polyglycolic acid sheet and fibrin glue, because it was observed only from the parenchyma of the interlobar surface. There were no intraoperative complications.
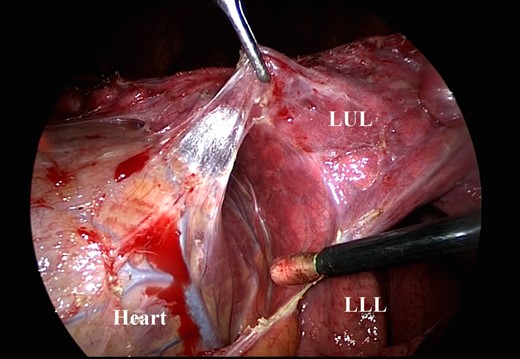
After the dissection between the heart and the lung, the epicardium is seen. LUL, left upper lobe; LLL, left lower lobe.
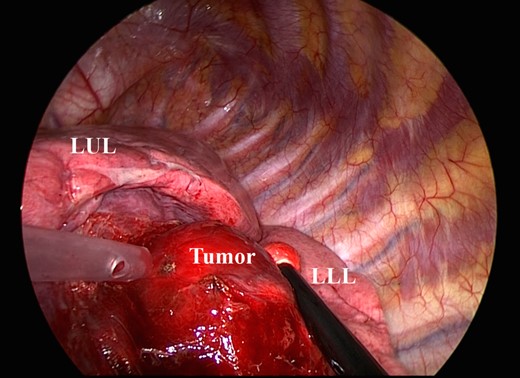
The tumor is located predominantly in the lower lobe, over the left major fissure, and spreads to the left upper lobe. LUL, left upper lobe; LLL, left lower lobe.
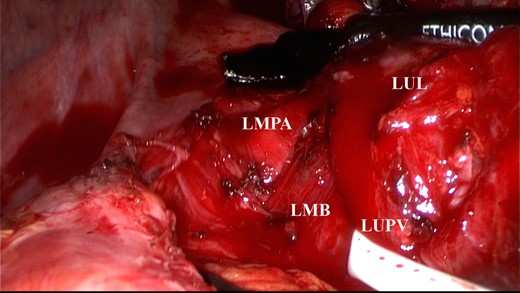
The left upper pulmonary vein was encircled and pulled caudally. The left main PA was exposed. LMPA, left main pulmonary artery; LUPV, left upper pulmonary vein; LMB, left main bronchus; LUL, left upper lobe.
The postoperative course was uneventful. The chest tube was removed on the fourth postoperative day (POD), and the patient was discharged on the seventh POD. The tumor was diagnosed as a pulmonary sclerosing hemangioma (PSH). The patient has survived with no signs of recurrence.
DISCUSSION
Lung surgery after cardiac surgery often requires the difficult process of dissection of adhesions in the pleural cavity. Shah et al. [1] reported a technique for performing thoracoscopic left upper lobectomy in patients with a previous left internal mammary artery. Concomitant cardiac and pulmonary surgery may allow us to avoid it; however, in our patient this did not apply. In our other cases, the previous cardiac surgery had caused adhesions of varying degrees; however, none of them presented with a defective pericardium. In our case, the pericardium had not been closed during cardiac surgery in infancy. We concluded that the heart had been gradually exposed as the patient grew up, because an unclosed pericardium does not close spontaneously.
The first successful case of arterial switch surgery for transposition of the great arteries was reported by Jatene et al. [2]. The PA trunk was transected and brought anteriorly to the aorta during surgery. Therefore, it was reasonable that postoperative inflammatory changes and fibrosis occurred around it. Thus, we could resect the tumor without bleeding from the interlobar PA. However, we thought that taping the PA was essential to perform safe enucleation.
The reason for operating on this patient was that we could not rule out malignancy because of the enlargement in tumor size and abnormal uptake on FDG-PET. We could have the choice of endobronchial ultrasound-transbronchial needle aspiration biopsy to confirm the preoperative diagnosis of the tumor, but the patient expected to have the resection of the tumor. Therefore, we decided to perform surgery for both diagnosis and treatment. We selected tumor enucleation due to intraoperative pathological findings and the location of the tumor. Postoperative permanent pathology results suggested PSH, but if the tumor had been malignant, we would have performed additional resection. If we had confirmed preoperative diagnosis as PSH, we would have chosen surgical treatment similarly because PSH has the potential to become malignant tumor, and some authors have reported recurrence and metastasis of a benign PSH tumor [3–6]. However, it is generally agreed that PSH does not affect the prognosis [3–6]. As it is difficult to make a precise preoperative and intraoperative diagnosis, there is no consensus on the optimal extent of resection [7]. We decided that periodical follow-up was needed instead of additional resection.
As a result of improved congenital cardiac surgery, it is expected that video-assisted thoracoscopic surgery (VATS) after congenital cardiac surgery will increase in the future. To perform VATS safely, general thoracic surgeons should notice what kind of an adhesion the previous cardiac surgery causes in the pleural cavity.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- pericardial sac
- transposition of great vessels
- pulmonary artery
- cardiac surgery procedures
- arterial switch operation
- adhesions
- follow-up
- infant
- surgical procedures, operative
- thoracoscopy
- heart
- neoplasms
- pathology
- pleura
- surgery specialty
- cellular enucleation
- chest ct
- excision
- pulmonary sclerosing hemangioma
- enucleation procedure
- left lung



