-
PDF
- Split View
-
Views
-
Cite
Cite
Ali Kordzadeh, Jiten P. Kalyan, Attila Jonas, Muhammad A. Hanif, Ioannis Prionidis, Cryptogenic mycotic aneurysm of the superior mesenteric artery, Journal of Surgical Case Reports, Volume 2015, Issue 8, August 2015, rjv106, https://doi.org/10.1093/jscr/rjv106
Close - Share Icon Share
Abstract
Visceral artery aneurysms as a result of arterial degenerative disease are rare (0.1–2%), and superior mesenteric artery (SMA) accounts for 3.2% of all reported series. However, mycotic SMA aneurysms (SMAAs) are even rarer, and to the best of our knowledge, this is the first report of cryptogenic mycotic aneurysm of SMA by Enterococcus faecalis (EF). We report a case of 77-year-old man with 6-week history of supra pubic/left iliac fossa pain, weight loss and fever. The computed tomography demonstrated an incidental finding of 4.4 × 3-cm SMAA with no primary foci. The subsequent serology and specimen confirmed EF. Aneurysmectomy without bypass grafting along with antimicrobial therapy resulted in full recovery of the patient.
INTRODUCTION
Superior mesenteric and celiac artery aneurysm accounts for only 4–5.5% of all reported visceral artery aneurysms with an estimated incidence of 0.1–2% in the literature [1, 2]. Furthermore, the majority of the reported cases are degenerative in nature. Their mycotic (infective cause) occurrence is rare, and according to Patel and Johnston's classification, majority are the result of a primary local source and/or secondary septic embolization [3]. However, no report in the literature has explained their cryptogenic development superior mesenteric artery aneurysm (SMAA) as a result of Enterococcus faecalis (EF). Herein, we present a recent, successfully treated case of cryptogenic mycotic aneurysm (MA) of SMA by EF with short review of the literature.
CASE REPORT
A 77-year-old man with past medical history of atrial fibrillation, hypertension and diabetes mellitus (Type 2) presented to the emergency department with 6-week history of supra pubic/left iliac fossa pain and 14-kg weight loss. On examination, he was found to be a febrile with distended but nontender abdomen. His laboratory data showed a new onset normocytic anemia (Hemoglobin 7.0 g/l) and normal inflammatory markers (white blood cell count 7.6 × 109/l, C-reactive protein 22 mg/l). Due to the nonspecific nature of the abdominal pain and weight loss a computed tomography (CT) with contrast was performed. This demonstrated an incidental finding of a 4.4 × 3-cm SMAA. This was associated with mesenteric edema and stranding and multiple local lymphadenopathies (Fig. 1).
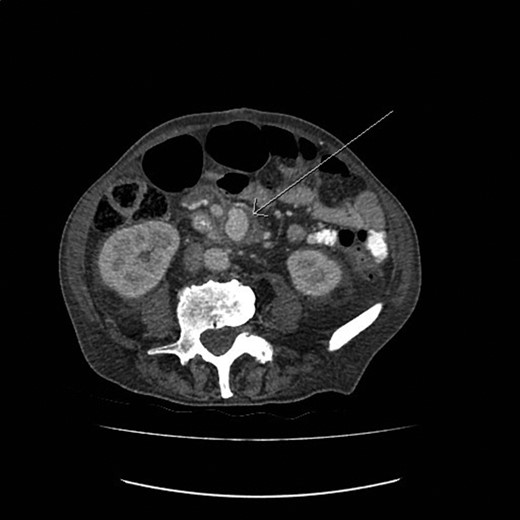
The CT image demonstrating a SMAA measuring 4.4 × 3 cm in size.
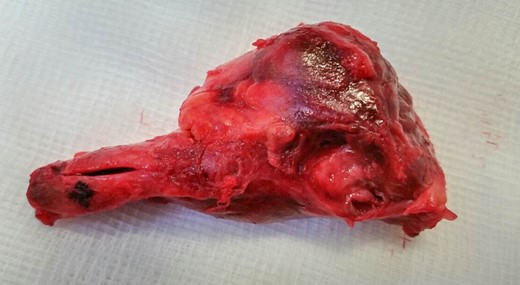
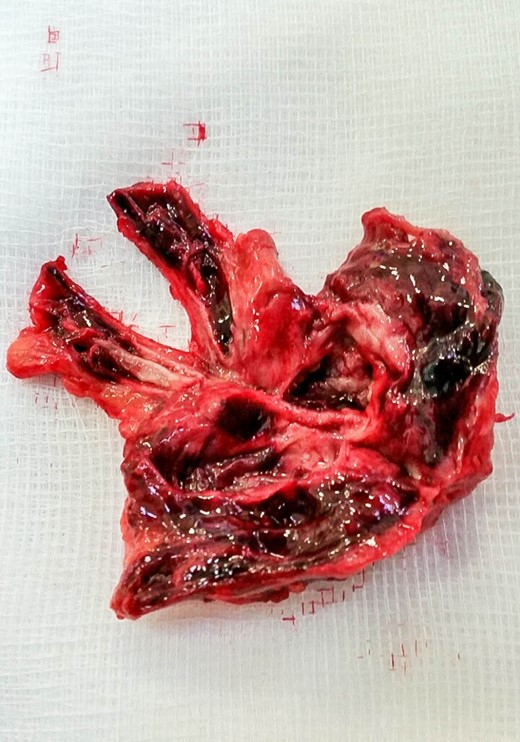
Exploratory laparotomy was performed, and after meticulous dissection, the proximal and distal ends of the SMAA were both isolated and clamped. The entire aneurysm and the surrounding inflammatory tissue, including lymph nodes, were fully excised (Figs 2 and 3). Due to good collateral flow (intestine), no bypass or interposition grafting was deemed necessary. Given the lack of any identifiable primary or secondary sources and normal echocardiogram, blood cultures were obtained and sent (along with the specimen) for histology and microbiology examinations. Subsequent blood culture revealed EF (Day 1) that was also later identified on the specimen. The histology demonstrated focal endothelial necrosis and neutrophil infiltration. The patient was immediately started on antibiotics (vancomycin) according to the sensitivity of the culture for a period of 6 weeks and discharged on the 6th postoperative day. Follow-up CT at 3 months demonstrated complete resection of the aneurysm with no inflammatory changes. The repeated blood culture and serology for inflammatory markers were normal, and the patient reported no complication on follow-up (Fig. 4).
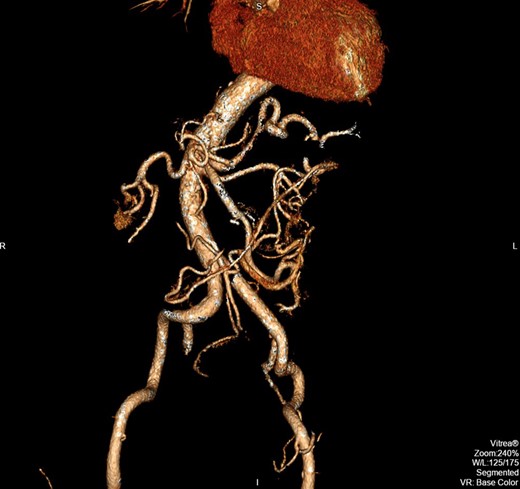
The postoperative CT image (3D reconstruction) demonstrating complete resection of the aneurysm.
DISCUSSION
EF is a gram-positive commensal bacterium that inhabits the gastrointestinal tract of humans. They are listed as the third leading cause of nosocomial infections and mainly affect immunosuppressed individuals [4]. They can cause meningitis, urinary tract and wound infections, but their cardiovascular involvement is rare and is limited to endocarditis (primary). Their sequelae can result in septic embolization (secondary), leading to MA of intracranial and coronary arteries (0.7–5.4%) [5, 6]. However, according to the literature, this sequence has led to two reported cases of secondary MA of SMA and no reported cases of cryptogenic development.
The cryptogenic development of MA is the result of viable circulatory organism that lodges in the arterial endothelium with no identifiable primary or secondary source [7]. The current literature suggests that abnormal intima can serve as a nidus for development of infection resulting in a cumulative endothelial injury, necrosis and formation of aneurysms. Intimal abnormality can occur in atherosclerosis, cystic medial necrosis, degenerative arterial disease, connective tissue disorders and noninfective inflammation of arteries [7]. The commonest location for such endothelial changes to act as a platform for development of MA by all organisms is femoral (38%), followed by abdominal aorta (31%), SMA (8%), brachial (7%) and iliac arteries (6%) [7].
Their management is complex, but bactericidal antibiotics over bacteriostatic are highly preferred and should be started as soon as the diagnosis is suspected. This regimen should not await micro-serological assay, and the optimal duration (following surgical intervention) of antibiotics is ≥6 weeks [7]. Due to high rates of mortality and morbidity as a result of rupture, surgical management in the form of excision and/or bypass is highly recommended [8]. If bypass is mandated, autologous graft over prosthetic conduits is advocated to avoid secondary infections and further incidence of re-intervention. In our reported case, due to good collateral flow no bypass or interposition grafting was necessary, but if advocated great saphenous vein, short saphenous vein and/or arm vein remain the best conduit of choice [8]. Another recently suggested approach is endovascular exclusion of SMAAs, but their long-term outcomes are awaited [9].
The investigative modality of choice is currently CT with contrast, and rarely any other investigative modality is required to complement this. Due to the vague and nonspecific presentation of such cases, a high index of suspicion and a low threshold for CT imaging are necessary [9].
In conclusion, MAs of SMA do not present with conclusive signs and symptoms for a clinical diagnosis. A CT scan, in such circumstance, can demonstrate important findings that might otherwise result in significant mortality or morbidity. Upon diagnosis, the aim should focus on their total excision or exclusion (endovascular). If bypass is granted, autologous graft is highly preferable and all patients should receive antibiotics for a period of ≥6 weeks irrespective of the surgical modality.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. No identification details have been used.
CONFLICT OF INTEREST STATEMENT
None declared.



