-
PDF
- Split View
-
Views
-
Cite
Cite
Edvin Prifti, Fadil Ademaj, Majlinda Ikonomi, Aurel Demiraj, Papillary fibroelastoma of the anterior leaflet of the mitral valve mimicking vegetation, Journal of Surgical Case Reports, Volume 2015, Issue 7, July 2015, rjv091, https://doi.org/10.1093/jscr/rjv091
Close - Share Icon Share
Abstract
The papillary fibroelastoma (PFE) is a rare and benign primary cardiac tumor, and the most frequently found tumor occurring in the cardiac valves. With the introduction of echocardiography, the diagnosis of these tumors in living patients has been reported sporadically. The PFEs have been found most often on valve leaflets, chordae tendineae, and both ventricles. We describe an interesting case of the PFE originating from the anterior leaflet of the mitral valve mimicking vegetation. The patient underwent successful surgical removal of the PFE.
INTRODUCTION
The papillary fibroelastoma (PFE) is a rare and benign primary cardiac tumor, and the most frequently found tumor occurring in the cardiac valves. PFE was firstly described by Yater in 1931 [1]. With the introduction of echocardiography, the diagnosis of these tumors in living patients has been reported sporadically. PFE has been found most often on valve leaflets, chordae tendineae, and both ventricles. We describe an interesting case of PFE originating from the anterior leaflet of the mitral valve mimicking vegetation.
CASE REPORT
A 52-year-old woman was readmitted to a regional hospital due to wound infection after a previous mastectomy due to breast cancer. She received antibiotics for 2 weeks to treat a suspicious vegetation apparent on transthoracic echocardiography (Fig. 1A). Because the size of the cardiac mass had not changed, the patient was referred to our hospital for further evaluation.
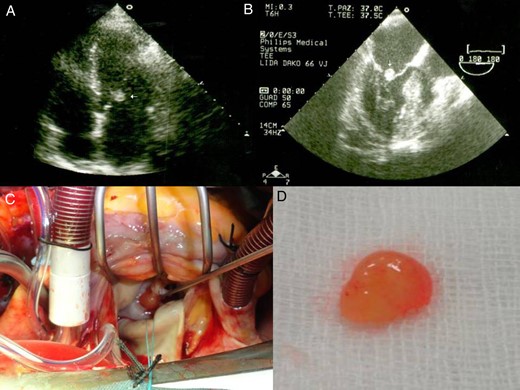
Transthoracic echocardiography shows a mobile and spherical mass of 1.0 cm in size attached to anterior mitral leaflet. Parasternal long-axis view (A) and an apical four-chamber view (B). Gross specimen of excised mass reveals a friable mass with frond-like surface.
On physical examination, her blood pressure, pulse and respiration rates were within normal values. The body temperature was 37.5°C. The laboratory findings revealed 15 700/mm3 leucytosis, platelet count 3 251 000/mm3 and erythrocyte sedimentation rate 53 mm/h. Results of urinalysis, blood chemistry and electrolyte tests were within normal ranges. TEE revealed a mass of >1.0 cm in size, mobile, attached to anterior mitral leaflet on the atrial aspect, and round with a homogenously speckled surface (Fig. 1B). There was moderate to severe mitral regurgitation.
The cardiac mass was removed by surgery in order to reduce the risk of embolism as well as to rule out the infective endocarditis. Under standard cardiopulmonary bypass, the left atrium was opened and the mass was identified (Fig. 1C). The mass was removed using a shave excision technique. However, there was significant mitral regurgitation after mass excision, and following excision of the mitral leaflets and chordae, a mechanical valve was implanted with preservation of the subvalvular apparatus of the mitral valve.
Macroscopically, the excised lesion was composed of a soft beige tissue with micropapillary projections and lobulated surface of 1.2 × 0.5 cm, entirely processed (Fig. 1D). The mass appeared yellowish, with polypoid and fibrotic characteristics. After the surgical excision, the mass was fixed in formalin, paraffin embedded, sectioned at 3 μm thick and stained conventionally with hematoxylin and eosin. The histology examination revealed a papillary lesion composed of numerous papillary fronds with an acellular fibro-hialinous stroma (Fig. 2A and B). Those projections are covered by endothelial cells (Fig. 2C). In the excised margin in close contact with the non-pathologic endocardial tissue, the lesion has an infiltrative-like appearance, but the excisional margin itself was free of neoplasia. A higher magnification demonstrates a myxoid papillary structure lined by endothelial cells that express endothelial cell markers (Fig. 2D). The diagnosis of PFE was confirmed. The postoperative course was uneventful, and the patient was discharged in a satisfactory condition on the seventh day.
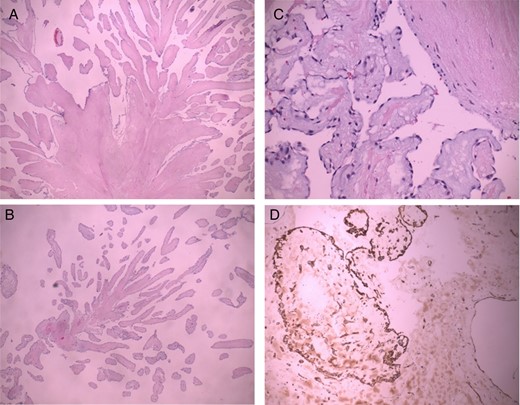
PFE. Low magnification demonstrates a papillary configuration of paucicellular fronds (A, B H-E 10×). A higher magnification demonstrates a myxoid papillary structure lined by endothelial cells that express endothelial cell markers (C H-E 40×, D CD 34 20×).
DISCUSSION
Different mechanisms such as prior damage to the endothelium, hamartomatous origin and organizing emboli to cause PFE [2]. If trauma is mechanically induced, PFE can occur in proximity to the iatrogenic injury [3]. The presence of fibrin and elastin fibers within the fronds supports the hypothesis that PFE may arise from organizing thrombi [2]. One of the most discussed theories is the microthrombus theory which stresses the fact that these lesions are acquired and they originate as small thrombi that serve as a nidus for further ecrescents to minor endothelial site injury of the valves or to previous diseased valves. PFE is considered also a hamartomatous growth of endocardial tissue that may give rise to neurologic symptoms when located on the left side of the heart. PFE is similar to Lambl's excrescences, but the site may be anywhere on the endocardium, and the lesion is large enough to potentially cause symptoms by embolization of attached thrombi or prolapse into a coronary orifice [4, 5]. The other theory is that PFEs are true tumoral lesions that predominantly affect heart valves.
The differential diagnosis of PFE includes other cardiac tumors, thrombus, vegetation and Lambl's excrescences. One of the most considered differential diagnoses is cardiac myxoma. In contrast to myxoma, there are no vascular structures and associated inflammation within the fronds. PFE has several characteristic findings that may help to differentiate it from thrombus [4] or vegetation [5]. Similar to vegetations, PFE is usually found on cardiac valves; however; PFE is often a solitary mass, usually of small size (<1 cm), usually at the mid-portion of valve leaflets and with a frond-like characteristic surface. However, PFE may lack some of these findings, whereas vegetations may represent many of these characteristics. For that reason, PFE can often be differentiated by clinical information, blood cultures and laboratory tests. In this case, there was evidence of wound infection, so the clinical information was quite misleading.
Although PFEs are benign, they can cause serious complications such as thromboembolism, myocardial ischemia, stroke and sudden death [6]. The systemic embolism is more frequently found in PFE of the mitral valve [7]. Surgical excision of PFE is recommended for symptomatic patients and asymptomatic patients with a fragile mass nature and frond-like papillary tissues of the tumor itself [8, 9].
Most PFEs are asymptomatic. Right-sided PFEs are asymptomatic and rarely cause pulmonary embolism [10], whereas left-sided can cause life-threatening complications. Patients might present chest pain, which may be anginal or atypical. Acute myocardial infarction, caused by a tumor occluding the coronary ostium or by embolization, may be the presenting symptom [10]. Cerebral embolization, either of fibrin or of a tumor fragment, has also been reported; this may present as a transient ischemic attack or visual disturbance.
Considering the patient's age, tumor mobility and tumor size, we decided to remove the cardiac mass to prevent potential complications such as embolization. The treatment of choice for PFE is surgical excision, which is safe without causing significant morbidity or mortality. When valvular involvement is present, excision with valve repair or replacement is curative.
CONFLICT OF INTEREST STATEMENT
This report has received the ethics committee approval. We confirm that we have obtained consent to publish such an information from the patient to report individual patient data. None of the authors have competing interests with the manuscript.



