-
PDF
- Split View
-
Views
-
Cite
Cite
Martin A. Nzegwu, Emmanuel Sule, Joseph Uzoigwe, Franklyn Achi, Metaplastic breast carcinoma; melanocytic variant, a very rare tumour, Journal of Surgical Case Reports, Volume 2015, Issue 2, February 2015, rju158, https://doi.org/10.1093/jscr/rju158
Close - Share Icon Share
Abstract
Metaplastic breast carcinoma (MBC) is a rare heterogeneous malignancy, accounting for <1% of all invasive breast carcinomas, in which adenocarcinoma is found to coexist with an admixture of spindle, squamous, chondroid or bone-forming neoplastic cells. Melanocytic variant was first described by Ruffolo et al. in 1997. We report a case of MBC, melanocytic variant, in a 57-year-old Nigerian female who presented with a left breast mass 8 cm in diameter in the upper outer quadrant, hard and gradually increase in size to become painful. Breast examination showed gross asymmetry. Left breast was oedematous and shiny with extensive peau d'orange. No palpable axillary nodes were seen. Chest X-ray and abdominal ultrasound scan showed no involvement. Breast biopsy revealed an invasive metaplastic ductal carcinoma with melanocytic differentiation.
INTRODUCTION
Metaplastic carcinoma of the breast is a rare heterogeneous malignancy, accounting for <1% of all invasive breast carcinomas [1]. Here, ductal carcinoma is found to coexist with an admixture of spindle, squamous, chondroid or bone-forming neoplastic cells [1]. Metaplastic breast carcinoma (MBC) comprising both epithelial and melanocytic elements is rare [1, 2]. It was first described by Ruffolo et al. in 1997; just seven cases of the melanocytic variant have been reported so far [1, 2]. Most common metaplastic breast cancers are oestrogen receptor (ER), progesterone receptor (PR) and Her2-neu negative [1, 3, 4], and they tend to have a worse prognosis than other triple-negative breast cancers [5, 6] with fewer therapeutic options. In this review, we discuss the histopathology and clinical features of MBC, melanocytic variant and their relevance to emphasize its diagnostic and management challenges.
CASE REPORT
A 57-year-old woman presented with 5/12 history of left breast lump which gradually increase in size and became painful. Menarche was at 13 years without oral contraception. She had four confinements, the first being 20 years. Each child was breastfed for 8 months. No family history of breast cancer. Clinical examination showed breast asymmetry. Left breast was oedematous, shiny with extensive peau d'orange. Patient applied herbal preparations with multiple superficial skin excoriations. Outer quadrant breast mass was 8 cm, hard, with attachment to skin and underlying structures. No palpable axillary lymph node was felt and axillary ultrasonography, though desirable, was not done. Liver was not enlarged. Chest X-ray and abdominal ultrasound scan showed no involvement. Initial breast biopsy revealed an infiltrating atypical spindled tumour with hyperchromatic nuclei, which was both S100 and tyrosinase-positive. Tumour fungated with a darkly pigmented surface despite two cycles of neoadjuvant doxorubicin-based chemotherapy. Left simple mastectomy (Fig. 1) and axillary sampling (Fig. 2) of the sentinel and another slightly enlarged node were done, revealed a triple-negative invasive ductal carcinoma (Figs 3–6), with melanocytic differentiation (Figs 7 and 8), positivity for S-100 and patchy positivity for tyrosinase, an enzyme involved in melanin formation from dihydroxyl phenyl alanine. Only the sentinel node was involved, with the other showing reactive changes. Core biopsy of the tumour had ductal elements and was triple-negative.

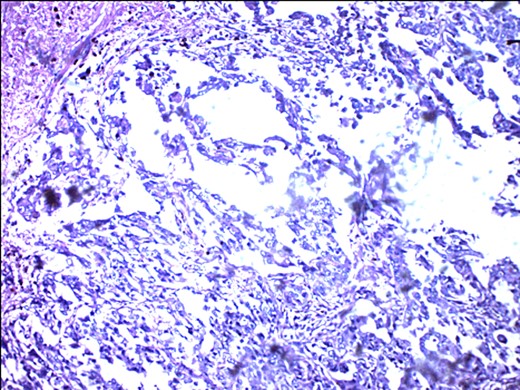
Metastasizing glandular component of MBC, melanocytic variant, with few pigment deposits.
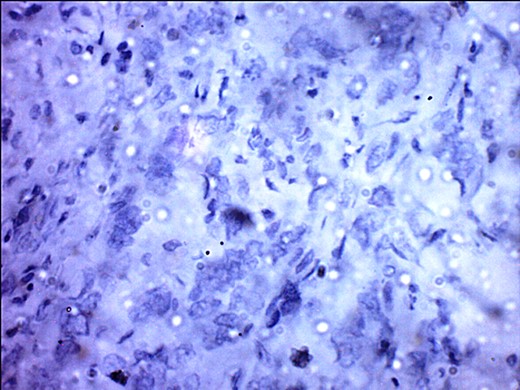
ER negativity for rabbit monoclonal antibodies. Few stromal cells are positive black.
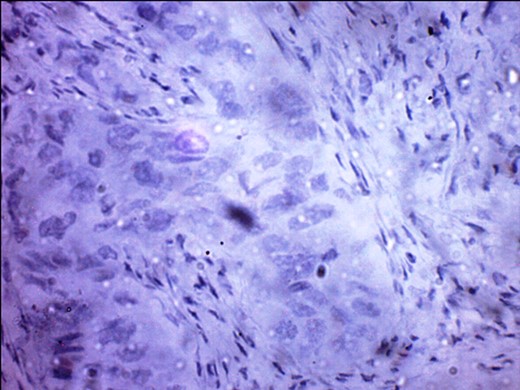
PR-negative immunohistochemistry typing with rabbit progesterone antibodies.
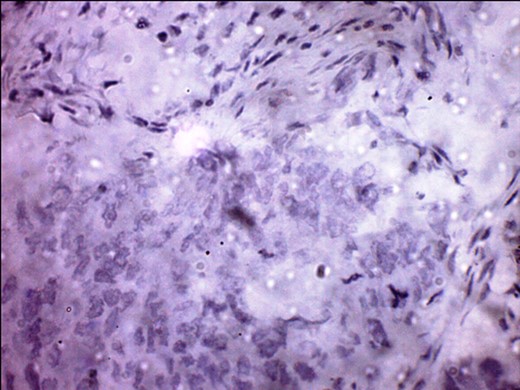
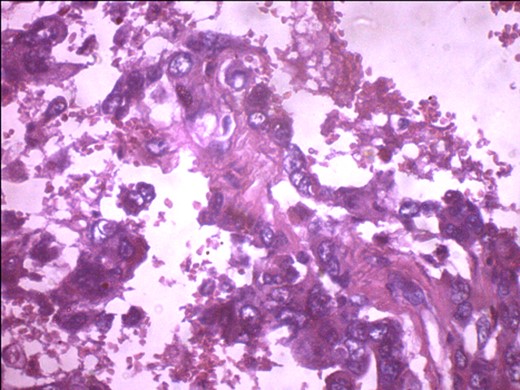
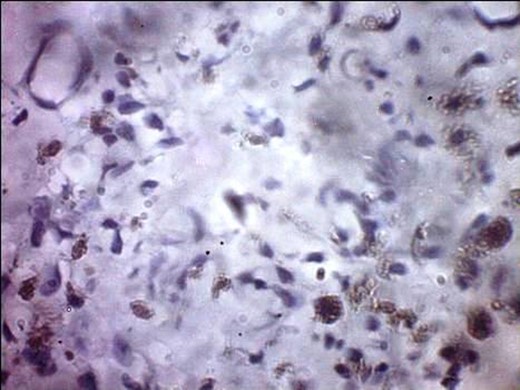
S-100 cytoplasmic positivity in keeping with melanocytic differentiation. Immunohistochemistry.
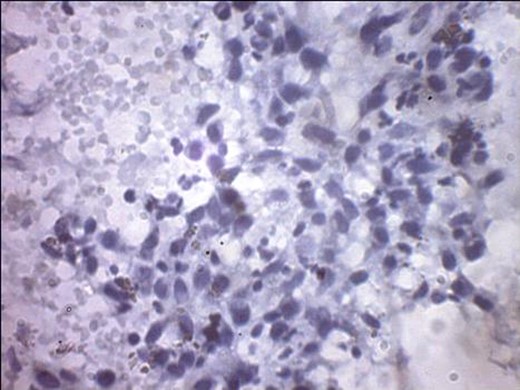
Tyrosinase positivity in keeping with melanocytic differentiation.
DISCUSSION
MBC is very rare, relative to invasive ductal carcinoma [1]. Our case of a melanocytic variant of metaplastic breast cancer represents the only documentation in medical literature from Nigeria and indeed Africa to our knowledge. In reports of MBC, all have been females [7] as in our case. Adher et al. has reported a median age of presentation of 48 years from Saudi Arabia [7]. Oberman [8] had earlier published a range of 14–58 years in a series from the USA. Our patient's age 57 years was in keeping with most reports that MBC is likely to occur in women older than 50 years [7, 8]. The mean tumour size reported was 9 cm with a range from 3 to 18 cm [7], consistent with the tumour size of our case. Clinical non-palpation of axillary lymph nodes at the first clinical assessment, despite the degree of local advancement, is consistent with reports of a low incidence of axillary metastases [7].
The mastectomy specimen had a tumour that measured 3 cm in its longest diameter with obvious black pigments. We noted two involved axillary nodes and skin involvement. Although MBC patients typically present with more advanced tumours relative to invasive ductal carcinoma [9], this could also represent the typical presentation for invasive ductal carcinoma in Nigeria from delayed presentation because our population represents a poorly screened population in terms of mammography mainly due to the absence of a viable health insurance scheme that addresses cancers as a whole.
Mastectomy was done in our case given the degree of local advancement. This is consistent with reports demonstrating higher employment of mastectomy compared with breast-conserving therapies in these patients [9].
There are reports that these tumours tend to be chemoresistant [10]. Hennessy et al. and Rayson et al. have both reported a dismal response to chemotherapy from 13 metastatic cases despite 10 different regimens being utilized with just one partial response [10]. In our case, response to neoadjuvant chemotherapy was partial with some clinical response with the first neoadjuvant cycle decreasing breast swelling and pain.
One of the universal finding in all studies is the high rate of ER/PR negativity with a reported range of 70–100% [6]. This is not unexpected because these tumours typically are basal like consistent with our report. Triple negativity of our case with a non-response to hormonal therapies as has added to the dilemma in the management of these patients, as no role has been demonstrated for them in these patients. Lymph node involvement is typically as described with the carcinomatous variant instead of the metaplastic variant [7] as seen in our report.
Initial chemotherapeutic regimen instituted was anthracyline, although cisplastin regimen is advocated as superior for basal-type tumours, especially MBC [10]. We observe the patient while she undergoes further chemotherapy and radiation treatments. There is a 17% response with platinum-based chemotherapy [10].
In conclusion, this rare report underscores the universality of this very rare entity and the fact that all physicians should have a high index of suspicion to diagnose the lesion early enough.



