-
PDF
- Split View
-
Views
-
Cite
Cite
Kateki Vinod, Vicente Diaz, Use of amniotic membrane graft in the surgical management of cicatricial ectropion associated with cetuximab therapy, Journal of Surgical Case Reports, Volume 2015, Issue 1, January 2015, rju143, https://doi.org/10.1093/jscr/rju143
Close - Share Icon Share
Abstract
Cetuximab (Erbitux) is an antiepidermal growth factor receptor (EGFR) monoclonal antibody that has been shown to delay the progression of metastatic colorectal cancer. The cutaneous side effects of cetuximab resulting from its effects on normal epidermal cells are well established. Periocular side effects, including blepharitis, trichomegaly, dry eye and conjunctivitis, have also been reported. We present a case of cicatricial ectropion associated with cetuximab therapy successfully managed with surgical repair using amniotic membrane graft. A 60-year-old man presented with bilateral lower eyelid cicatricial ectropion developing 3 days after the addition of cetuximab therapy to his baseline chemotherapeutic regimen. This was successfully managed with surgical repair using an amniotic membrane graft. Surgical repair with the amniotic membrane graft is a viable treatment option for cicatricial ectropion associated with EGFR inhibitor therapy.
INTRODUCTION
Cetuximab (Erbitux) is a monoclonal antibody that binds to tumor cells expressing epidermal growth factor receptor (EGFR), thereby disrupting abnormal cellular processes like cell proliferation, inhibition of apoptosis, metastasis and angiogenesis [1]. Cetuximab has been associated with both cutaneous and periocular side effects. We report a case of cicatricial ectropion associated with cetuximab therapy for metastatic colorectal cancer.
CASE REPORT
A 60-year-old man with colorectal cancer metastatic to the liver, lungs and lymph nodes presented with a 3-day history of irritation and redness of bilateral lower eyelids. Review of systems was also notable for other integumentary symptoms, including skin breakdown near the nails and a skin rash on the upper extremities and back. His symptoms began 3 days after the addition of cetuximab infusions to his baseline chemotherapeutic regimen of 5-fluorouracil (5-FU)/leucovorin, which he had been receiving for 8 months. At the time of presentation, the patient had been managed with artificial tears, with no relief of his symptoms.
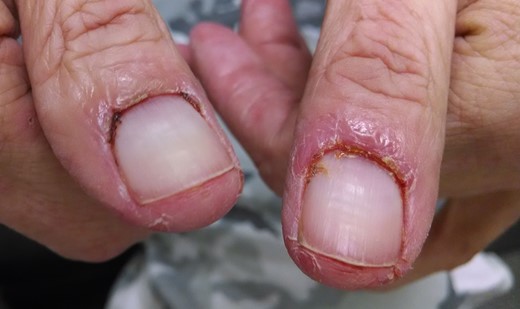
On examination, the patient had normal visual acuity, pupillary responses, extraocular motility and intraocular pressures. External examination revealed cicatricial ectropion of bilateral lower eyelids with associated madarosis and lagophthalmos (Fig. 1). The surrounding periorbital skin was thickened and taut. The remainder of his anterior segment examination was within normal limits, including white and quiet conjunctivae and clear corneas bilaterally. The dilated fundus examination was also within normal limits. Integumentary examination further revealed paronychia and a maculopapular follicular rash on the upper extremities and back (Figs 2 and 3).
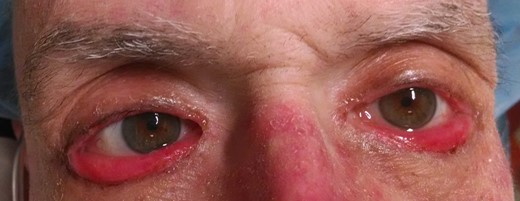
External photograph demonstrating cicatricial ectropion of bilateral lower eyelids associated with cetuximab use.
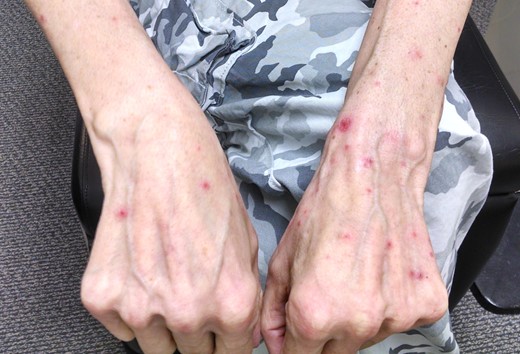
Maculopapular follicular rash of the upper extremities associated with cetuximab use.
After providing informed consent, the patient was taken to the operating room for repair of cicatricial ectropion of bilateral lower eyelids. The surgical procedure consisted of draping amniotic membrane graft over the lower palpebral conjunctiva, and securing it to the deep fornix with sutures that were externalized to the skin surface and secured with bolsters.
As a result of cutaneous and periocular side effects, the patient's oncologist discontinued cetuximab and initiated therapy with docetaxel. At his postoperative week 1 visit, the patient described resolution of ocular symptoms and ophthalmic examination revealed improved lower eyelid height and contour (Fig. 4).
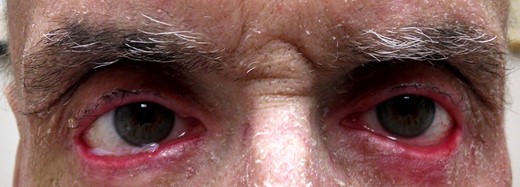
External photograph demonstrating improved lower eyelid height and contour 1 week after surgical repair of bilateral cicatricial ectropion with the amniotic membrane graft.
DISCUSSION
Cetuximab is an EGFR inhibitor indicated for the treatment of metastatic or unresectable colorectal cancer and squamous cell tumors of the head and neck [1]. EGFR is expressed in normal epidermis, sweat glands and hair follicles, and its inhibition by cetuximab has been associated with numerous cutaneous side effects [2]. In a series of 14 patients by Santiago et al. [2], adverse cutaneous reactions following cetuximab therapy included acneiform skin rash, paronychia, xerosis, and change in hair texture and growth.
Periocular side effects, such as blepharitis, trichomegaly, dry eye and conjunctivitis, have also been reported, though less frequently [2–7]. Among these, trichomegaly is the most common, and can be complicated by trichiasis and corneal erosion [8]. Review of the literature revealed only one previously reported case of cicatricial ectropion associated with cetuximab therapy, in which discontinuation of cetuximab resulted in resolution of periocular signs and symptoms 6 weeks later [9].
Our case report and review of the oncologic and ophthalmic literature underscore the importance of recognizing the potential ocular and periocular side effects of cetuximab therapy. Patients undergoing systemic chemotherapy who develop signs of ocular toxicity would benefit from early referral to ophthalmologists, who can manage the ocular side effects in conjunction with oncologists. Patients with cicatricial ectropion may be managed conservatively with cessation of cetuximab when possible, or surgically with amniotic membrane grafts especially when continuation of cetuximab is essential to their oncologic treatment regimen. The success of amniotic membrane grafts has been demonstrated previously in the management of the cicatricial complications relating to inflammatory disorders of the eyes and eyelids, such as Stevens-Johnsons syndrome, by acutely suppressing inflammation and promoting epithelial healing [10]. Our results suggest that surgical repair with amniotic membrane grafts may offer the potential for more rapid resolution of cicatricial ectropion associated with cetuximab therapy—as early as 1 week postoperatively—than cessation of cetuximab alone.
CONFLICT OF INTEREST STATEMENT
None declared.



