-
PDF
- Split View
-
Views
-
Cite
Cite
Sean Bennett, Mindy Lam, Jason Wasserman, David Carver, Navaaz Saloojee, Terence Moyana, Rebecca A. Auer, John Lorimer, A case series of two glomus tumors of the gastrointestinal tract, Journal of Surgical Case Reports, Volume 2015, Issue 1, January 2015, rju144, https://doi.org/10.1093/jscr/rju144
Close - Share Icon Share
Abstract
Two recent cases of glomus tumors (GTs) of the gastrointestinal tract presented with symptoms of GI bleeding. GTs, typically benign lesions of mesenchymal origin, are rarely seen in the GI tract, and most commonly involve the distal appendages. This case series discusses the tumor biology, presentation, imaging, endoscopic findings, pathology and management of GTs. While diagnosis of GTs is typically made on final surgical pathology, there are defining characteristics that can separate a GT from a gastrointestinal stromal tumor on endoscopic ultrasound (EUS) and CT imaging. The classic pathological findings are discussed, and surgical decision-making is reviewed. New developments in the form of EUS-guided biopsy and endoscopic submucosal dissection present new avenues for diagnosis and treatment of submucosal lesions of the GI tract, including GTs. While typically a benign tumor requiring no adjuvant therapy, this study discusses some very rare cases of metastatic GT in the literature.
INTRODUCTION
The glomus body is an arterio-venous shunt involved in thermodynamic regulation [1]. They are most numerous in the distal appendages where they play a role in heat exchange. The majority of glomus tumors (GTs), which are mesenchymal in origin, are therefore found in the hands and feet. Rarely, GTs are found in the GI tract, primarily in the stomach [2]. The incidence of gastric GTs is 100× lower than gastrointestinal stromal tumors (GISTs) [1]. In the largest study of gastrointestinal GTs (32 cases), Miettinen et al. found a female preponderance of 72%, and median age of 55. Of the 31 patients with gastric GTs, 11 presented with upper GI bleeding, 9 with dyspepsia and 1 with perforation. Six tumors were found incidentally during surgery for another reason, or endoscopy. The only non-gastric GT was in the cecum, and presented as appendicitis [3].
CASE REPORT
An 88-year-old female presented with 5 days of weakness and dyspnea, severe anemia (hemoglobin of 46 g/l), and no evidence of bleeding on history. Physical examination was remarkable for a blood pressure of 83/48, and black stool on rectal examination. Endoscopy demonstrated a 4-cm submucosal mass in the distal body of the stomach. The mass had central ulceration and a visible vessel, not actively bleeding. The presumptive diagnosis was GIST. CT demonstrated a 2.5 × 1.9 cm well-circumscribed, partially exophytic, arterially enhancing lesion. She underwent wedge resection of the greater curvature. She was discharged home on postoperative day 3 without complication. Pathology found a lobulated tumor composed of glomus cells in the submucosa and muscularis propria. The cells were positive for α-smooth muscle actin, caldesmon and vimentin. There was focal positivity for synaptophysin and CD34. These are consistent with a GT.
A 70–year-old male presented with light headedness, shortness of breath and 2 days of bright red blood per rectum progressing to melena. His hemoglobin was 92 g/l, a decrease from 144 g/l 10 days prior. Upper endoscopy demonstrated a 3-mm polyp in the duodenal cap (biopsy would be reported as a completely excised carcinoid tumor). Colonoscopy demonstrated a bleeding, edematous region in the ascending colon. Biopsy was non-diagnostic. CT demonstrated a 2.3 × 1.6 cm ascending colon lesion with hyperenhancement in the arterial phase and persistent enhancement in the portal venous and delayed phases. On octreotide scan, the lesion had intense uptake, consistent with a carcinoid. The clinical picture was in keeping with two synchronous primary carcinoid tumors. Laparoscopic converted to open right hemicolectomy was performed without complication. Final pathology demonstrated a GT with negative margins, 26 negative lymph nodes, 1 mitosis per 50 hpf, no vascular invasion and absence of necrosis. Follow-up for the colonic GT will include a colonoscopy and abdominal CT 1 year following surgery, as well as chromogranin A level every 6 months. Chromogranin A was mildly elevated preoperatively. For his duodenum, he will be followed with Esophagogastroduodenoscopy +/− endoscopic ultrasound (EUS) every 3–6 months initially.
DISCUSSION
Endoscopic findings of gastric GTs are that of a submucosal mass, typically in the antrum or distal body, with either normal mucosa or ulceration [3, 4] (Fig. 1). CT shows a well-defined submucosal tumor with a clear margin, strong enhancement in the arterial phase, and prolonged enhancement in the delayed phase [4, 5] (Fig. 2). Often confused with GIST, the distinguishing features are that the density of GISTs is lower than that of GTs, and GISTs do not exhibit prolonged enhancement in the delayed phase [5]. On EUS, the GT is found in the fourth endosonographic layer, with a heterogeneous appearance. In one small study of seven gastric GTs, six demonstrated the characteristic ‘peripheral halo’ sign around the tumor [5].
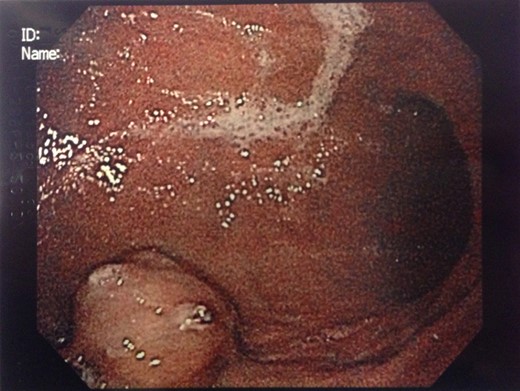
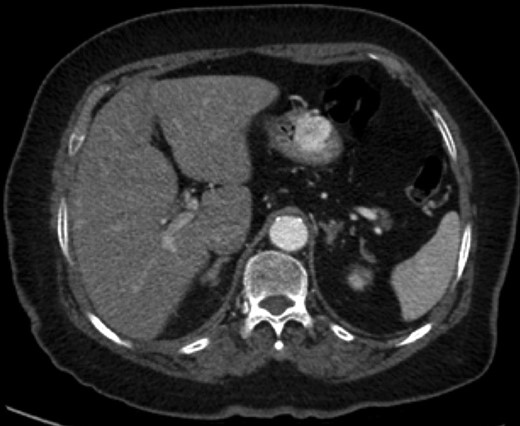
Endoscopic biopsies of submucosal lesions are typically superficial and non-diagnostic. However, having a preoperative diagnosis could lead to a change in surgical planning, given the benign nature of GTs. EUS-guided biopsy is now a well-established modality for sampling submucosal lesions [6]. There are case reports in the literature of EUS-guided biopsy being diagnostic for GTs [7]. This could potentially spare an extensive surgical resection.
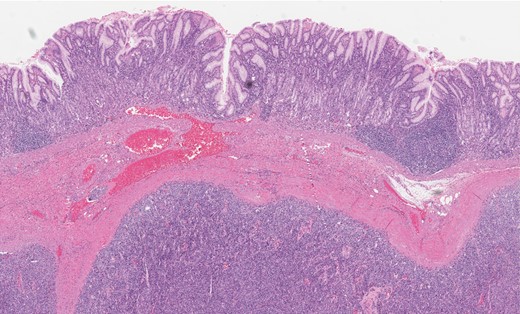
Gastric GT. Note the well-circumscribed submucosal lesion surrounding small vessels (H&E stain, ×20).
Grossly, GTs are multinodular, soft and rubbery on sectioning [3]. The cellular nodules are separated by streaks of gastric smooth muscle, which also surrounds the tumor [3]. Histologic features are central round to oval nuclei with inconspicuous nucleoli and clear to eosinophilic cytoplasm with distinct cell borders [1] (Figs. 3 and 4). GTs are positive for α-smooth muscle actin, vimentin, calponin and caldesmon. They are most often negative for CD117, CD34, chromogranin and synaptophysin [3, 6].
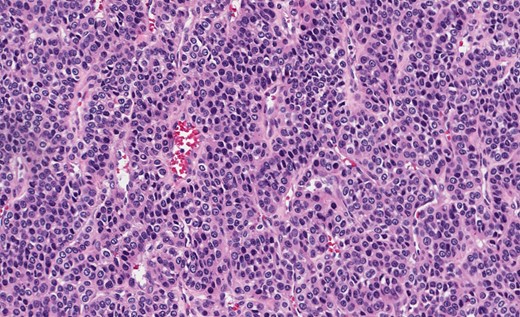
Gastric GT. Note central round or oval nuclei with clear, eosinophilic cytoplasm (H&E stain, ×200).
GTs can present with chronic bleeding, often occult. While life-threatening hemorrhage is rare, severe anemia is seen. There is minimal literature about colonic GTs; however, one patient in the Miettinen study presented as appendicitis secondary to a cecal GT.
The disease most often follows a benign course; however, there are very rare case reports of metastases. One 65-year-old female was found to have a gastric GT with synchronous metastases of the kidney, brain, lung and humeral head. She died 7 months after initial diagnosis [8]. Another describes a 58-year-old male who presented with cutaneous metastases to the scalp 6 years after gastrectomy for a GT [9]. Staging revealed lung, liver and brain metastases, and subsequent cutaneous nodules. He received palliative radiotherapy for symptomatic skin lesions, and died soon after.
Typical management is local resection without adjuvant therapy. Given their benign course and small size, gastric GTs are amenable to wedge resection [3]. There are limited reports of endoscopic submucosal dissection (ESD) being used in gastric GTs. Zhou et al. [10] described 26 gastric submucosal tumors treated with ESD, 3 of which were GTs. They had no serious adverse events. No residual tumor or recurrence was found after a mean follow-up of 8 months. For symptomatic colonic GTs, an appropriate resection with lymphadenectomy should be done. It seems reasonable to suggest regular follow-up with investigations based on symptomatology. In cases with high mitotic activity, there may be a role for endoscopic and CT surveillance.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- glomus tumor
- biopsy
- endoscopy
- gastrointestinal bleeding
- decision making
- surgical pathology
- surgical procedures, operative
- tourette syndrome
- diagnosis
- diagnostic imaging
- neoplasms
- pathology
- gastrointestinal tract
- benign neoplasms
- gastrointestinal stromal tumor
- adjuvant therapy
- subepithelial lesions
- endoscopic submucosal dissection
- glomuvenous malformations
- endoscopic ultrasound



