-
PDF
- Split View
-
Views
-
Cite
Cite
Louay Habbab, Haifa Alfaraidi, Andre Lamy, Surviving catastrophic disintegration of a large left atrial myxoma: the importance of multi-disciplinary team, Journal of Surgical Case Reports, Volume 2014, Issue 9, September 2014, rju093, https://doi.org/10.1093/jscr/rju093
Close - Share Icon Share
Abstract
Atrial myxomas are the most common primary cardiac tumors, representing ∼50% of all benign cardiac tumors. Patients with a left atrial myxoma (LAM) generally present with symptoms of mechanical obstruction of blood flow, systemic emboli or constitutional symptoms. Embolic complications may occur any time with progression of the tumor; therefore, myxoma is usually considered an indication for urgent surgery. This report describes a patient with mobile large LAM who survived multiple emboli to the brain, spleen, kidneys, abdominal aorta and lower limbs during hospitalization for surgery, illustrating the critical nature of this finding and its possible catastrophic complications and demonstrating the importance of multi-disciplinary team in the decision-making process and the management of such complications and supporting the hypothesis that intravenous thrombolysis may be safely used in the treatment of embolic stroke due to cardiac myxoma.
INTRODUCTION
Atrial myxomas are the most common primary cardiac tumors, representing ∼50% of all benign cardiac tumors [1]. Patients with a left atrial myxoma (LAM) generally present with symptoms of mechanical obstruction of blood flow, systemic emboli or constitutional symptoms [2]. Systemic embolization usually results from necrotic tumor fragments or thrombi from the surface [2] and rarely from complete tumor detachment [3]. This report describes a patient with large mobile LAM who survived catastrophic multiple embolization to the brain, spleen, kidneys, abdominal aorta and lower limbs, illustrating the critical nature of this finding and demonstrating the importance of multi-disciplinary team in the management of such complications.
CASE REPORT
A 52-year-old female was referred to our hospital with the diagnosis of LAM. She had pneumonia 3 months prior to admission. The diagnosis of myxoma was made during the workup of shortness of breath that persisted despite the treatment of the pneumonia with antibiotics. The patient was a heavy smoker. On admission the patient appeared ill with a blood pressure of 130/86 mmHg and a heart rate of 105/min. Clinical examination revealed a mid-diastolic murmur increasing on expiration with a grade 2/6 pan systolic murmur located at the sternal border increasing on inspiration and bilateral basal crepitations on chest auscultation. Chest X-ray showed some atelectasis with right blunting of the costophrenic angle. ECG showed sinus rhythm with ST segment depressions in the lateral leads.
Transthoracic echocardiogram (TTE) revealed a 4.8 × 2.5 cm mobile mass arising from the interatrial septum of a severely dilated left atrium, causing moderate left ventricular inflow obstruction with a mean gradient of 12 mmHg and trace mitral regurgitation (Fig. 1A and B). There was severe tricuspid regurgitation with a systolic pulmonary artery pressure of 61 mmHg. The patient described symptoms of angina before her current hospitalization for shortness of breath. For that reason, a coronary angiogram was ordered before her surgery and revealed 80% lesions in the mid-left anterior descending artery and a 70% stenosis at the origin of the posterior descending artery of a dominant left circumflex artery.
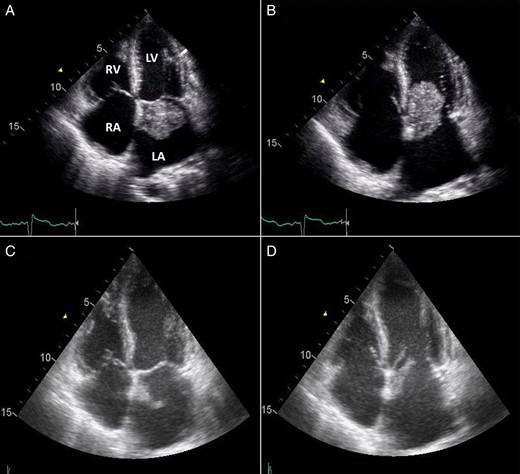
TTE 4-chamber view during systole (A) and diastole (B) showing a large LAM attached to the interatrial septum and obstructing diastolic filling of the left ventricle and follow-up four-chamber view during systole (C) and diastole (D) showing a marked reduction in the size of the LAM (LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle).
The next morning after her coronary angiogram, the patient suddenly showed signs of confusion and began to experience slurred speech and left-sided facial droop with left-sided hemiplegia. She was intubated to protect the airway and was sent for an urgent computed tomography (CT) scan of the head, which revealed a hyperdense minute clot lodged at the right internal carotid artery bifurcation. Accordingly, tissue plasminogen activator thrombolytic therapy was initiated. A repeat TTE showed that the mass was markedly decreased in size (2.1 × 0.9 cm) compared with a previous study, likely due to embolization (Fig. 1C and D).
On further examination, her legs were cold and mottled and a CT angiography of the abdomen, pelvis and both lower extremities revealed occlusion of the distal infra-renal abdominal aorta and iliacs (Fig. 2A and B) and superficial femoral arteries with weak runoff on the left and no runoff on the right side (Fig. 2C and D). Multifocal infarcts of the spleen and both kidneys were also noticed (Fig. 2A).
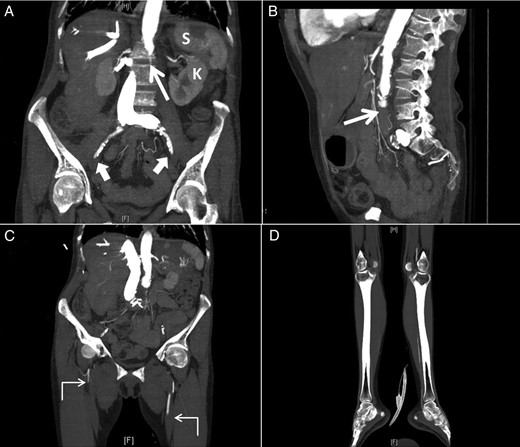
Sagittal (A) and coronal (B) computed tomographic angiogram images of the abdomen and pelvis and coronal images (C and D) of both lower extremities showing occlusion of the distal infra-renal abdominal aorta (thin arrow) and iliacs (thick arrows) and superficial femoral arteries with weak runoff on the left and no runoff on the right side (angled arrows) with multifocal infarcts of the spleen (S) and left kidney (K).
Repeated CT scan of the head 24 h later confirmed the diagnosis of an evolving large right hemisphere infarction involving both anterior and middle cerebral arteries territory with midline shift (Fig. 3A and B). Hemicranial decompression was performed and a follow-up CT scan of the head, 24 h later, showed transcranial herniation of the right cerebral hemisphere with reduction of the midline shift (Fig. 3C and D). Likewise, because of the multiplicity of the emboli, she also developed occlusion of the aorta as well as in both femoral systems and right popliteal artery that required corresponding embolectomies and bilateral aortofemoral bypass surgery. The pathological examination of the emboli confirmed the diagnosis of atrial myxoma.
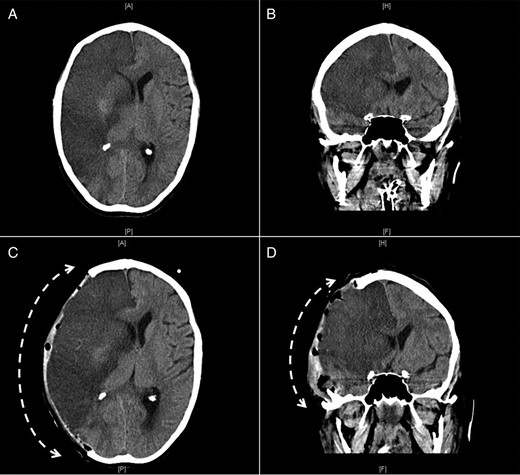
Axial (A and C) and coronal (B and D) CT images of the brain showing a large infarct in the area of the right middle cerebral artery before (A and B) and after (C and D) right hemicranial decompression with transcranial herniation of the right cerebral hemisphere (interrupted curved arrows).
After a stormy postoperative course, the patient was left with substantial neurological deficits caused by right hemisphere damage including dense left-sided hemiplegia, speech articulation problems and swallowing deficits that required percutaneous endoscopic gastrostomy tube insertion. Few months later and following intensive medical management that included cranioplasty utilizing the original skull fragment preserved after the craniectomy, the patient was discharged to be followed by rehabilitation services and to be evaluated for surgical removal of the remnant of the LAM as soon as her general condition allows.
DISCUSSION
This patient represents a typical cardiac myxoma case, occurring in a woman between the third and sixth decades of life, localized to the left atrium and arising from the interatrial septum, which is the case in 75–90% of the cardiac myxomas [4]. The patient gave a 3-month history of not feeling well with persistent SOB and later developed multiple systemic embolizations and all these findings are expected with LAM. Occurring in 67% of the cases, intracardiac mechanical obstruction of blood flow is the commonest cause of most presenting symptoms of LAM including dyspnea, palpitation, chest discomfort, dizziness and syncope [1, 2]. Systemic embolization occurs in 30–40% of patients [1, 2], with 50–70% of embolic episodes affecting the central nervous system [5]. In this report, the patient suddenly ruptured her large mobile LAM and developed multiple emboli to the brain, spleen, kidneys, abdominal aorta and lower limbs. Such presentation illustrates the critical nature of this finding and its possible catastrophic complications and demonstrates the importance of multi-disciplinary team in the decision-making process and the management of such complications. It also supports the hypothesis that intravenous thrombolysis may be safely used in the treatment of embolic stroke due to cardiac myxoma [6].



