-
PDF
- Split View
-
Views
-
Cite
Cite
Sara Nasser, Christian von Heymann, Aarne Feldheiser, Ute Schäfer-Graf, Iris Klempert, Alexander Pöllinger, Florian Krackhardt, Wolfgang Henrich, Jalid Sehouli, Klaus Pietzner, A rare case of ovarian cancer in pregnancy complicated by pulmonary embolus and myocardial infarction: management dilemmas, Journal of Surgical Case Reports, Volume 2014, Issue 10, October 2014, rju099, https://doi.org/10.1093/jscr/rju099
Close - Share Icon Share
Abstract
Malignant ovarian neoplasms diagnosed during pregnancy at advanced stages are very rare. The clinical course and prognosis of pregnant patients diagnosed with epithelial ovarian cancer is similar to that of non-pregnant patients. We describe our management of a woman diagnosed with FIGO IIIc ovarian cancer at Caesarean section. Immediately after surgery she suffered a pulmonary embolus and a myocardial infarction. She showed signs of a severe pulmonary hypertension (59 mmHg). Four weeks later the pulmonary hypertension was still moderate but, despite her critical status, she underwent primary debulking surgery (PDS). This was performed under extensive anaesthesiological monitoring. Through this rare case, we show that despite the complex initial status of a critically ill patient, PDS can still remain the mainstay of treatment in patients with advanced ovarian cancer as most patients are able to tolerate even extensive debulking surgery without the need for neoadjuvant chemotherapy.
INTRODUCTION
Primary ovarian cancer in pregnant women is very rare [1]. Only 2–3% of all ovarian tumours in pregnancy are malignant and fewer than 20% of epithelial ovarian cancers (EOCs) occur in premenopausal women [2]. Contrary to ovarian cancer in non-pregnant women, it is rarely diagnosed in advanced stages (Stages III–IV), as a result of routine ultrasound examinations during pregnancy. The clinical course and prognosis of pregnant patients with EOC is similar to that of non-pregnant patients [2].
We describe our management of a patient diagnosed with Stage IIIc epithelial ovarian carcinoma at Caesarean section. This was further complicated by a para-neoplastic thromboembolic myocardial infarction and pulmonary embolism.
CASE REPORT
A 34-year-old woman was referred following an emergency Caesarean section at 38 + 4 weeks.
Her antenatal screening tests were unremarkable. Intraoperatively both ovaries appeared tumorous. Biopsies confirmed a serous papillary ovarian adenocarcinoma. Further inspection of the intraperitoneal cavity was not performed. Retrospective blood analysis showed an elevated CA-125 of 99.8 (Normal <35).
She received thromboembolic prophylaxis on admission. Shortly after, she developed syncopal episodes. ECG findings and rising cardiac enzymes confirmed a myocardial infarct. Computed tomography (CT) scan showed evidence of a pulmonary embolus. In light of the above, the patient was transferred to the intensive care unit and received therapeutic anticoagulation. Subsequently, a coronary angiography revealed a thromboembolic closure of the distal right coronary artery (Fig. 1) which was treated with partial thrombectomy. The patient remained stable but her echo-cardiogram showed severely raised pulmonary artery pressure (59 mmHg).
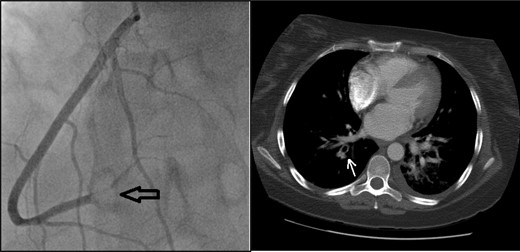
Coronary angiogram (left): note the visible blockage in the distal right coronary artery (black arrow). CT thorax (right): pulmonary embolus in the subsegments of the right upper lobe (white arrow).
A repeat echo-cardiogram showed moderate regression of pulmonary hypertension (33 mmHg). The case was presented at the multidisciplinary tumour conference to discuss neoadjuvant chemotherapy versus primary debulking surgery (PDS). Due to the improving pulmonary hypertension, the team decided to perform PDS. The patient gave informed consent and underwent median incision laparotomy 6 weeks after her diagnosis.
The surgery consisted of a hysterectomy, bilateral salpingo-oophorectomy (BSO), omentectomy, pelvic and paraaortal lymphadenectomy and deperitonealization with optimal tumour debulking.
Intraoperatively, there was minimal ascites. The tumorous left ovary was adherent to the uterus and lateral pelvic wall (Fig. 2).
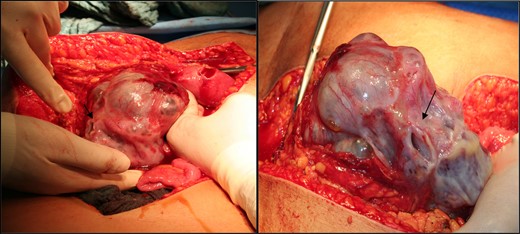
Intraoperative picture of the tumour mass. Note the spontaneous rupture site denoted by the arrow.
Diffuse tumour nodes were visible in the omentum. Colon, small bowel, diaphragm, liver and spleen showed no macroscopic metastasis. The hysterectomy, BSO and pelvic deperitonealization were resected en bloc (Fig. 3).
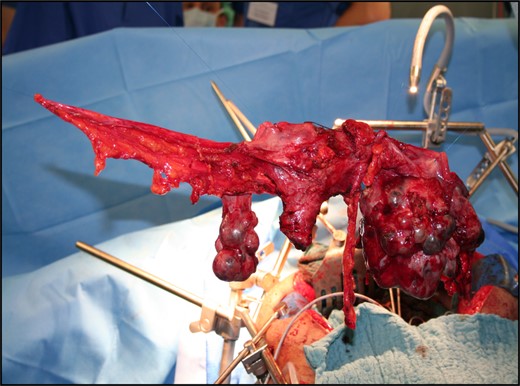
Arterial, central-venous and pulmonary artery lines were placed to monitor the pulmonary hypertension perioperatively and administer fluid and catecholamine therapy. Continuous norepinephrine was administered to maintain mean arterial pressure. Postoperatively, the patient spent 2 days in the high dependency unit prior to ward transfer.
Final histology confirmed a moderately differentiated serous adenocarcinoma in both ovaries with five positive pelvic lymph nodes, but no malignant cells in the ascites.
The final staging was FIGO IIIc (pTIcpN1 (5/54) G2 L1). Following discharge the patient received adjuvant chemotherapy with carboplatin AUC5, paclitaxel 175 mg/m2 and bevacizumab 15 mg/kg.
DISCUSSION
Ovarian cancer is a rare finding in pregnancy [3] with an incidence between 0.073 and 0.08 in 1000 pregnancies.
There are no records of large randomized trials of pregnant women treated for ovarian cancer.
Prognosis of EOC in pregnancy is similar to that of non-pregnant patients and relies on the same factors [1, 4].
It is well established that optimal cytoreduction at primary surgery remains the single most important prognostic factor in ovarian cancer [5].
PDS remains the standard treatment for ovarian cancer [5–8]. It is only advantageous if it results in no or minimal residual tumour load [5, 8–10]. This is partly dependent on surgical experience. In fact, trials have shown that the rates of optimal PDS in small centres are around 20–30% compared with 60–90% in highly specialized gynaecological oncology centres [9]. Patient characteristics also influence resectability of widespread disease [10].
In an attempt to increase the proportion of patients that are left with no or minimal disease, the concept of neoadjuvant chemotherapy followed by interval debulking surgery has been developed. The proposed advantages of this approach are a higher rate of optimal resection, less extensive surgery and stabilizing patients prior to surgery.
In our case, we favoured PDS despite the extensive anaesthetic input due to several reasons. First, the literature provides no evidence to the superiority of neoadjuvant chemotherapy to PDS in improving overall patient survival rates. Vergote et al. [5] compared PDS with neoadjuvant chemotherapy in patients with stage IIIC–IV ovarian cancer in a randomized trial of 632 patients. He found that overall survival rate (29–30 months) and progression-free survival (12 months) were similar in both the primary surgery and chemotherapy groups.
Secondly, a potential drawback of neoadjuvant chemotherapy is that the occurrence of fibrosis and the presence of smaller tumour masses after the treatment may make identification and complete resection of macroscopic disease difficult [5]. The biological basis of PDS states that small tumours have more central blood perfusion and a higher percentage of dividing cells [10] favouring diffusion of cytotoxic drugs. Hence, cytoreductive surgery will increase the susceptibility of smaller lesions to chemotherapy compared with large tumour masses. These smaller lesions will also require fewer chemotherapy cycles to be eradicated, thus lowering the risk of developing drug resistance [10].
In our case, the patient was admitted to a specialized centre where the rate of optimal surgical cytoreduction is very high. Furthermore, she had no other risk factors apart from her recent thromboembolic events. Her pulmonary pressure improved during her admission and she remained haemodynamically stable. These factors favour PDS over neoadjuvant chemotherapy based on the previous arguments. In light of the above, we performed PDS through extensive anaesthetic management despite the patient's critical condition, instead of administering neoadjuvant chemotherapy, as this ultimately provides the best possible survival outcome.
Overall, the management of pregnant women with a malignant ovarian tumour is similar to what is recommended for non-pregnant patients. Maximal primary cytoreductive surgery remains the standard care for women with advanced ovarian cancer. Patients with advanced stage ovarian cancer should be treated in specialized centres to maximize the chances of residual-free tumour debulking during primary surgery. This case report highlights the rarity of EOC in pregnant women and the advantages of performing PDS even in critically ill patients with extended anaesthesiological monitoring without the need for neoadjuvant chemotherapy.



