-
PDF
- Split View
-
Views
-
Cite
Cite
Senthurun Mylvaganam, Rebecca Taylor, Steven Thrush, Poly implant breast implants (PIP) and the rupture risk in asymptomatic patients: a warning for greater clinician suspicion in assessment and counselling, Journal of Surgical Case Reports, Volume 2013, Issue 2, February 2013, rjs044, https://doi.org/10.1093/jscr/rjs044
Close - Share Icon Share
Abstract
Poly implant prostheses (PIP) implants have raised concern due to the increased reporting of ruptures prompting a UK review in 2012 recommending that symptomatic patients only undergo magnetic resonance imaging (MRI) and explantation as necessary. Literature suggests two of three implant ruptures are asymptomatic. In this case, a 45-year-old woman with bilateral breast implants for cosmesis presented due to publicity of PIP implants. She was asymptomatic with no clinical signs suggestive of rupture. Due to patient intention for explanation of rupture, an MRI scan was undertaken which showed extensive silicone between the chest wall and lung bilaterally. The lung multidisciplinary team did not recommend removal of the lung silicone infiltrate. The implants were removed confirming rupture. Symptoms and signs cannot be relied upon to diagnose implant ruptures. Where patient concern exists with expressed intent for explantation if proven rupture, MRI assessment is a reliable tool where clinical uncertainty over rupture is present.
INTRODUCTION
All breast implants are regulated under the European Union Medical Devices Directive and are implemented in the UK by the Medicines and Healthcare Products Regulatory Agency (MHRA). Poly implant prostheses (PIP) implants received approval in 2000 and gained widespread use in the UK from 2001. From 2001 to 2009, ∼80 000 implants were sold in the UK. Over 95% of these implants were provided for cosmetic reasons. In 2008, MHRA raised concerns regarding the increased reporting of ruptures of PIP implants, but it was not until 2010 that the French regulator Agence Francaise de Securite Sanitaire des Produits de Sante (AFSSAPS) discovered the use of industrial rather than medical grade silicone in PIP implants and the product lost its European approval.
In December 2011, following a large increase in the reported rupture rate and a possible cancer risk, the French ministry for health recommended consideration of PIP explantation. This prompted a Department of Health UK expert review to quantify health risk and provide recommendations for UK patients and health professionals [1].
The review in January 2012 concluded that there were no data to suggest that PIP implants were associated with a higher risk of breast cancer. There is evidence though to suggest that the gel in PIP implants is less cohesive and more likely to spread locally on rupture and generate an increased inflammatory response [2]. The expert panel concluded that there was no quality data to compare rupture rates of PIP against other implants. However, they did recognize that under reporting may be a major factor in PIP as they have been mainly used in the private sector where in the UK reporting to the regulator is voluntary and also often patients do not have routine follow-up where adverse events can be identified.
The recommendations for UK patients and health professionals from the Keogh review are that routine explantation of PIP implants is not recommended. However, patients should be given relevant information and advice if they have a PIP implant and only subject to clinical need will further procedures be offered. All patients where PIP implants were offered as part of NHS treatment would be contacted to inform them that they have the implant and provided with relevant information and advice. General practitioners were advised to refer patients for specialist opinion if they present with signs and symptoms to include breast lumpiness, axillary lymphadenopathy, breast redness or tenderness, pain or hyperaesthesia. The imaging of choice by the specialist would then be an MRI and if a rupture is confirmed the NHS will offer explantation and only if the implants were placed by the NHS will they also be exchanged [1]. However, the Keogh report does recognize that up to two in three implant ruptures may be subclinical, potentially leaving many patients with undiagnosed implant ruptures [1].
The following case reports an asymptomatic patient with PIP implants and reflects on what can be learned for all clinicians.
CASE REPORT
A 45-year-old female presented to the breast clinic with concerns over bilateral breast implants which she had implanted over 10 years ago. These had been carried out under the NHS, but she had had no follow-up since and wondered whether these should be removed given recent publicity regarding PIP implants.
She had neither noticed any change in the shape of her breasts nor any lumps, although she had experienced some mild breast pain recently (possibly related to cyclical hormonal changes). On examination, the implants were non-tender but appeared to have migrated laterally towards the axilla. There was no axillary lymphadenopathy. There was no clinical suggestion of rupture or leak.
With the patient keen for explantation if there was proven rupture, an MRI scan was organized as the recommended imaging modality. This showed no silicone in the breast or axilla. However, there was extensive silicone between the chest wall and the lung. Although there was no definite sign of intracapsular rupture, the appearances were suggestive of a gel bleed. These findings were seen bilaterally; however, they were worst on the right side as shown in Figure 1a and b.
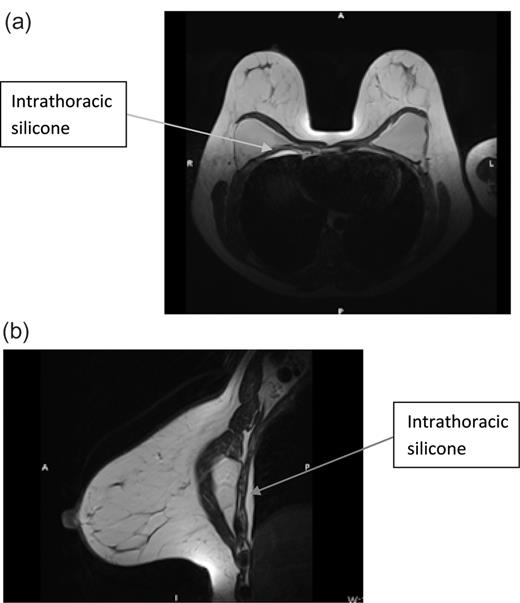
(a) MRI breast cranio-caudal view and (b) MRI breast medio-lateral view.
A respiratory opinion concluded that intrathoracic removal of the silicone was not recommended. However, following a discussion with the patient, it was decided to remove the implants to prevent further leakage.
DISCUSSION
Although it was not clear whether this patient had PIP implants, this clinical scenario is becoming a more common phenomenon facing GP's, breast and plastic surgeons. Where there is doubt with regards to the nature of the breast implant, clinical diligence and patient anxiety demands they are assumed to be PIP and so have a potentially increased risk of leakage. The final UK department of health report on PIP implants concluded that they are twice as likely to rupture as other implants with a rate of 6–12% at 5 years and 15–30% at 10 years [3]. In addition, it has been recognized that most implant ruptures do not produce any symptoms and signs. This silent rupture does produce limitations in providing a true rupture rate as only those women seeking imaging will be diagnosed. PIP implants though are more likely than other implants to produce symptoms with the rate of explants with signs of 0.7 at 5 years and 1.9% at 10 years [4]. The evidence available with regards to PIP implants will continue to evolve with time, but current UK recommendations and guidance on risk and assessment of rupture may underestimate and miss patients with subclinical implant ruptures as highlighted in this case. Although PIP implants have no quality data to compare the rupture rates compared with other contemporary implants, possibly due to under reporting, the trend suggests that they are at an increased risk of rupture. It is also suggestive that upon rupture they are more likely to spread locally and generate a greater inflammatory response. This case highlights the possibility of widespread implant rupture in an asymptomatic patient and hence the need to consider radiological assessment in all patients to complement the clinical assessment by breast and plastic surgery specialists.
Patients with PIP implants reviewed by a breast specialist in the absence of symptoms should be counselled with regards to the possibility of implant rupture. Following counselling with regards to the patients' views on explantation, particularly if the implant was provided outside of the NHS where there will be no offer of exchange, a patient decision should be reached on whether the patient would consider explantation despite being asymptomatic if rupture is present. Only where the patient would have explantation without NHS implant replacement, asymptomatic patients should undergo radiological assessment. Radiological assessment can be performed by ultrasound (US) or MRI, with the literature supporting MRI as the more accurate method for identification of breast implant rupture [5]. MRI certainly has a greater sensitivity for detecting rupture whilst the specificity may be argued to be similar with reported US sensitivity and specificity of 30–50 and 81–83%, respectively, and MRI sensitivity and specificity 64–90 and 77–90%, respectively [6,7]. In this case, MRI was also able to quantify the extent of silicone spread in the thorax which US may not have been able to. Hence, MRI can be a better compliment clinical assessment to detect rupture in the asymptomatic patient.
Conflict of interest statement. None declared.



