-
PDF
- Split View
-
Views
-
Cite
Cite
Winson Jianhong Tan, Claramae Shulyn Chia, Hock Soo Ong, A rare cause of gastrointestinal haemorrhage: gastric invasion by hepatocellular carcinoma, Journal of Surgical Case Reports, Volume 2013, Issue 1, January 2013, rjs050, https://doi.org/10.1093/jscr/rjs050
Close - Share Icon Share
Abstract
Patients with hepatocellular carcinoma (HCC) are predisposed to upper gastrointestinal (GI) haemorrhage with bleeding gastro-oesophageal varices and peptic ulcers being the common aetiologies. On rare occasions, HCC with direct invasion into the upper GI tract can lead to haemorrhage. Recognizing the possibility of invasive HCC causing upper GI haemorrhage is of paramount importance as acute management differs from the usual aetiologies. We describe a 76-year-old lady with long-standing liver cirrhosis who presented with upper GI haemorrhage due to an HCC invading into the greater curvature of the stomach. Trans-arterial embolization was performed which led to successful cessation of bleeding. Direct invasion of the GI tract by HCC causing haemorrhage is an extremely rare condition. Compared with endoscopic therapy, trans-arterial embolization offers the best chance of successful haemostasis and should be considered first-line therapy in these patients.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer-related mortality globally [1]. Gastrointestinal (GI) haemorrhage is a common complication in these patients. The usual aetiology is either due to gastro-oesophageal varices or ulcers. In rare instances, HCC invading into GI tract may be the cause of GI haemorrhage. We present a case of a 76-year-old lady with GI haemorrhage due to gastric invasion by HCC who was successfully managed with trans-arterial embolization.
CASE REPORT
A 76-years-old lady with background history of cryptogenic Child's B liver cirrhosis presented with intermittent epigastric pain of 2 weeks duration associated with abdominal distension and nausea. On examination, vital signs were stable but marked pallor was noted in her conjunctiva. Physical examination was remarkable for abdominal distension with mild splenomegaly and ascites. Rectal examination revealed brown stools.
Laboratory investigations revealed anaemia with haemoglobin of 6.8 g/dl. Liver function tests were unremarkable with the exception of low albumin of 33 g/l and mildly elevated aspartate transaminase of 46 U/l. Coagulation profile was mildly deranged with a prothrombin time of 11.3 s and a normal partial thromboplastin time. She was admitted under the gastroenterology service with the preliminary diagnosis of dyspepsia with anaemia for investigation. Two pints of packed cells were transfused.
During the second day of admission, the patient passed out large amounts of melena and had an episode of haematemesis. She also developed tacchycardia with heart rate increasing to 110–120 b.p.m. Repeat blood investigations showed that haemoglobin had fallen from 6.8 to 5.3 g/dl despite blood transfusion. An emergency oesophagogastroduodenoscopy was performed. Findings were that of grade 1 oesophageal varices with no features of variceal bleed. There was a large clot in the fundus and cardia of the stomach. After attempted removal of the blood clots with flushing, the patient developed torrential bleeding necessitating intubation for airway protection. The bleeding eventually ceased spontaneously and the source of bleeding was identified to be originating from a large ulcer crater in the greater curve with an overlying clot (Fig. 1). Endoscopic ultrasound was performed which showed a 10 cm mass lesion abutting the stomach wall and extending into the peritoneal cavity (Fig. 2). The preliminary impression was that of a bleeding GI stromal tumour and care was transferred to the surgical team for consideration of surgical intervention.
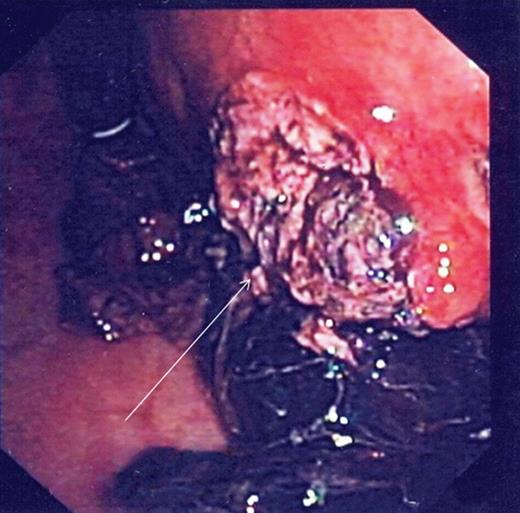
Endoscopy showing ulcer crater in greater curvature of stomach.
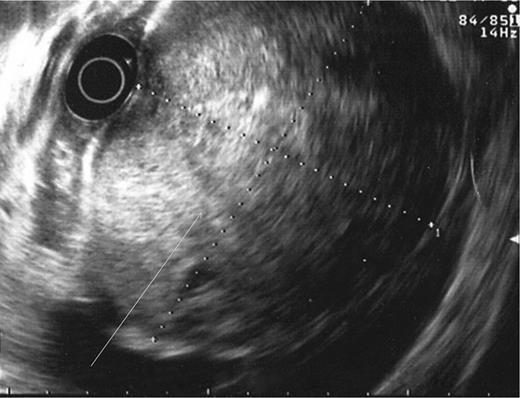
Endoscopic ultrasound showing lesion abutting stomach wall extending into peritoneal cavity.
A computed tomography scan was performed which showed multifocal HCC with a large exophytic lesion invading into the greater curvature of the stomach (Figs. 3 and 4). Peritoneal metastases and ascites were present. The patient underwent an angioembolization of the hepatic tumour on the same evening. Angiography identified a replaced left hepatic artery as the dominant vessel supplying the lesion of concern in the left lateral hepatic segment (Fig. 5a). Gelfoam embolization was performed with no residual tumour enhancement thereafter (Fig. 5b).
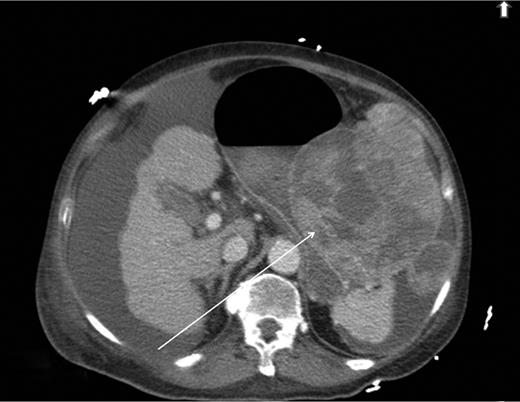
Computed tomography image showing HCC invading into greater curvature of stomach.
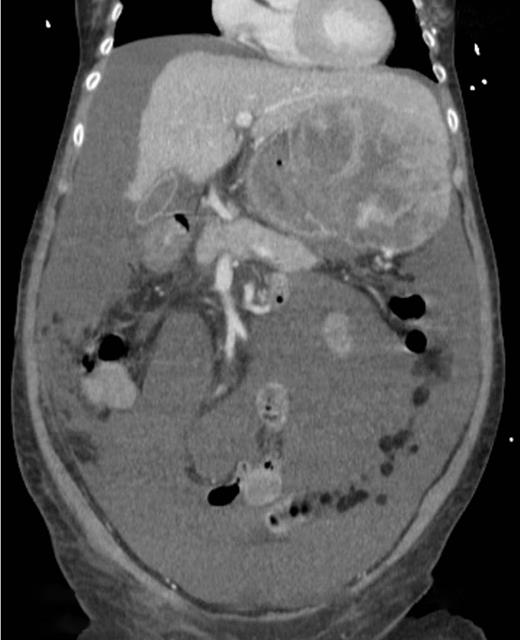
Computed tomography image showing tumour originating from the liver.
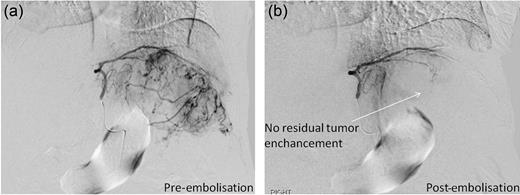
Pre- and post-trans-arterial embolization images of HCC leading to successful haemostasis.
The patient's bleeding ceased after intervention and her haemoglobin levels stabilized. Alpha-foetoprotein level was elevated at >60 500 μg/l. She was eventually discharged and will be reviewed by the medical oncologists for consideration of palliative chemotherapy.
DISCUSSION
Patients with HCC are predisposed to upper GI haemorrhage as they commonly have portal hypertension either due to underlying liver cirrhosis or due to portal vein involvement by tumour thrombus. Common causes of upper GI haemorrhage in HCC patients include bleeding gastro-oesophageal varices, peptic ulcers, congestive gastropathy and gastric erosions. On rare occasions, direct invasion of HCC into the upper GI tract can lead to haemorrhage. This accounts for only 5.5% of GI haemorrhage in patients with HCC [2]. Worldwide, <20 cases of GI bleeding from upper GI invasion by HCC have been reported [3]. Although uncommon, it is important to recognize hepatic tumour invasion as a possible aetiology of upper GI haemorrhage as management strategies differ significantly from the common aetiologies.
Diagnosis of upper GI invasion by HCC causing haemorrhage is difficult and can be easily missed. Radiological imaging may show a bulky HCC invading into the stomach or duodenum, but there have been numerous cases of upper GI tract involvement by HCC which were not demonstrated on imaging [4–6]. Hence, the absence of typical features of tumour invasion in imaging studies does not exclude the pathology. Compared with imaging, endoscopy may better visualize these lesions but HCC invasion of upper GI tract can adopt a myriad of appearances ranging from an ulcerative bleeding mass to a polypoidal or submucosal mass with or without features of extrinsic compression [5]. Thus, it requires a combination of imaging, suggestive endoscopic findings and a high index of suspicion before a diagnosis of HCC invasion causing upper GI haemorrhage can be confidently made. Endoscopic ultrasound may be a useful adjunct as demonstrated in our case. It identified the lesion to be originating from the peritoneal cavity which on hindsight should have led us to consider HCC invading into the stomach as a differential diagnosis in this patient with long-standing liver cirrhosis.
Optimal management of HCC invasion causing upper GI haemorrhage hinges on making the correct diagnosis. Biopsy of the lesion may lead to torrential bleeding and should be avoided if clinical suspicion of upper GI invasion by HCC is high. The options available in the acute setting include endoscopic interventions, trans-arterial angioembolization and surgery. In contrast to the other more common causes of upper GI haemorrhage, the chance of successful endoscopic haemostasis is low in these cases [5, 7, 8]. To the best of our knowledge, there has only been one case report whereby successful endoscopic haemostasis was achieved [8]. Trans-arterial embolization offers better results and should be considered first-line therapy to cease bleeding and stabilize the patient in the acute setting before considering definitive surgical resection in suitable candidates [5, 7, 9]. Although surgical resection may control bleeding, majority of patients with HCC invading into the GI tract have extensive tumours not amenable to curative resection. As palliative resection confers a dismal survival, surgery in the acute setting should only be considered as a last resort when other measures of achieving haemostasis have failed [5].



