-
PDF
- Split View
-
Views
-
Cite
Cite
T.S. Korampalli, Bipin Mathew, N.D. Stafford, Post radiation myofibrosarcoma of hypopharynx, Journal of Surgical Case Reports, Volume 2013, Issue 1, January 2013, rjs049, https://doi.org/10.1093/jscr/rjs049
Close - Share Icon Share
Abstract
Malignant tumours of myofibroblasts are rare lesions. Post radiation sarcoma is a rare potential late sequel of ionizing radiation. We present the first case of myofibrosarcoma of the hypopharynx in a 67-year-old man, occurring 16 years after radiotherapy for laryngeal carcinoma.
INTRODUCTION
Most malignant tumours in the hypopharynx are carcinomas. There are some reports of hypopharyngeal sarcomas such as angiosarcoma, liposarcoma and synovial sarcoma [1]. Ionizing radiation is an important modality of treatment for head and neck cancer. However, radiation-induced sarcoma of the head and neck is a rare long-term complication of radiation therapy.
We report the first case of myofibrosarcoma of the hypopharynx occurring 16 years after radiotherapy to a well-differentiated squamous cell carcinoma of larynx. Our objective is to highlight this rare clinicopathological entity to head and neck surgeons and histopathologists.
CASE REPORT
A 67-year-old man presented to our ENT department with history suggestive of globus feeling in the throat. The patient had received a full course of radiotherapy to the larynx for well-differentiated T1N0M0, squamous cell carcinoma of the larynx. There was no past history of hereditary conditions predisposing to sarcomas, including Recklinghausen's disease, hereditary forms of retinoblastoma and Wilms tumour. Direct laryngoscopic examination revealed a postcricoid tumour extending to the oesophagus. Biopsy was reported as a grade 2 spindle cell sarcoma. Computed tomographic scans demonstrated a post-cricoid lesion extending into the cervical oesophagus (Fig. 1). No cervical lymphadenopathy or distant metastases were noted.
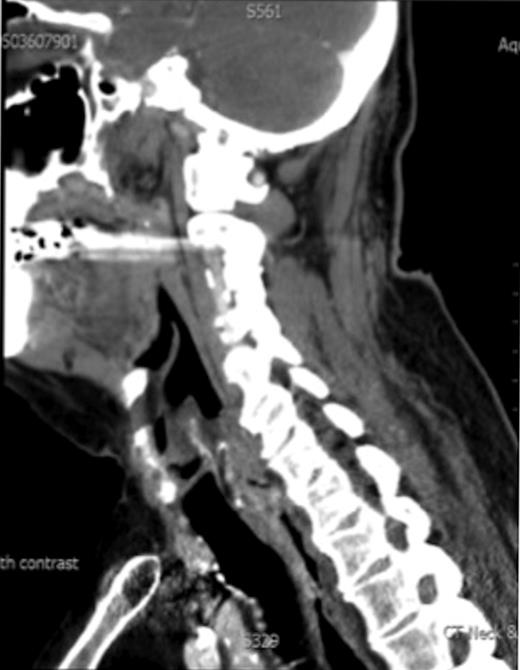
CT scan showing the post cricoid carcinoma extending down to upper oesophagus.
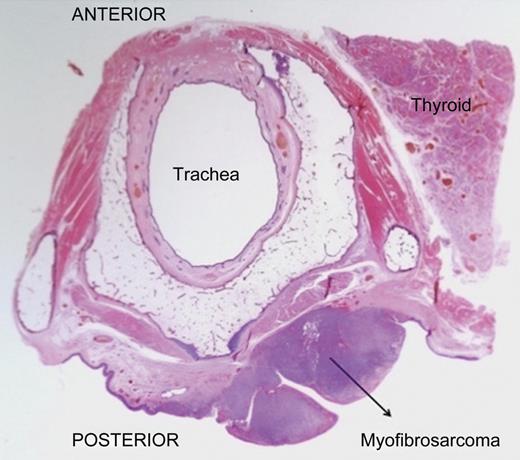
The patient underwent a total laryngopharyngectomy with flap repair. Macroscopically, the hypopharynx showed a polypoidal tumour measuring 50 × 32 × 21 mm in dimensions. Microscopy showed spindle cells arranged in short fascicles and sheets. Individual cells showed short spindle and elongated hyper-chromatic nuclei with mild-to-moderate amount of pale to eosinophilic cytoplasm (Fig. 2). There was no evidence of squamous or glandular differentiation. There was no dysplasia within the adjacent squamous epithelium. On immunohistochemistry, diffuse staining for smooth muscle actin (SMA) in a tram track pattern was noted. In addition, a minor population of tumour cells stained for desmin (Fig. 3). There was no staining for AE1 AE3, pancytokeratin, H-caldesmon, myogenin, CK 5/6, CK14, p63, 34BE12, CD34, CK7, CD56, EMA, S100, melan A, c-KIT and CD99. The final diagnosis of a myofibrosarcoma FNCLCC grade 2 was made. The surgical margins were free of tumour.
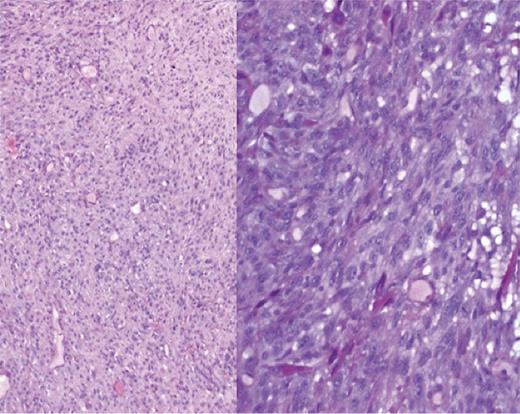
×5 and ×10 magnification shows a cellular tumour with short to elongated cells with scant intervening stroma.
DISCUSSION
Sarcomas are malignancies derived from the mesoderm. They account for <1% of head and neck malignancies and <10% of all soft tissue sarcomas [2].
Myofibrosarcoma was first reported in 1992 [3]. They are malignant tumours of myofibroblasts, with predilection for the head and neck region followed by extremities and trunk. They occur across a wide age range (9–75 years, mean age 40 years) with slight male predominance.
Radiotherapy plays a key role in the treatment of head and neck malignancy. Cumulative incidence of sarcoma after radiation therapy ranges from 0.03 to 0.3%, [4]. A significant proportion (16–36%) of these was malignant fibrous histiocytoma.
Cahan et al. developed the first set of criteria for a sarcoma to be appropriately identified as radiation induced. However, this applied only to bone sarcomas. Later, Murray et al. revised these to include other soft tissue sarcomas. Cahan et al. and Laskin et al. [5] modified these criteria: (i) a prior history of radiation; (ii) development of a sarcoma in the field of radiation; (iii) a latency period of at least 2 years between radiation and appearance of the sarcoma and (iv) evidence that the sarcoma is histologically different from the irradiated primary cancer. All the above criteria were satisfied in our case.
According to recent studies, the range of radiation doses associated with the development of post radiation soft tissue sarcomas is 8–100 Gy, with a mean dose from 40 to 50 Gy [6]. The latency interval has ranged from 2 to 65 years, but the mean latency is 15 years [6]. Sheppard and Libshitz found the latency interval to be inversely related to the radiation dose [7].
Post radiation sarcomas are aggressive, with a high incidence of local recurrence and distant metastasis [8]. A survival rate of 29% after 5 years was reported by Wiklund et al. [9]. Surgical resection is the preferred treatment when possible, on the basis of the site and size of tumour.
Histopathologically myofibrosarcomas are characterized by a cellular proliferation of spindle-shaped cells arranged in intersecting fascicles and sheets within scant collagenous to myxoid stroma. Mitotic activity is low and necrosis usually absent. Myofibrosarcoma reveals a smooth muscle actin and calponin-positive immunophenotype. In addition, a small proportion (<50%) of cells can stain for desmin. There can also be focal expression for H-caldesmon. These findings are useful in distinguishing myofibrosarcomas from leiomyosarcomas, which usually express both desmin and H-caldesmon in a large proportion of cells. Fibrosarcomas also fall in the differential diagnoses. However, these do not stain for smooth muscle actin and calponin.
Review of the literature suggests that the risk of post radiation sarcoma in the head and neck is extremely low. Mark et al. estimated the risk of post radiation sarcoma with long-term follow-up to be 0.03–0.8% [10] and the risk for radiation-induced sarcoma in head and neck appeared no worse in comparison with mortality risks from chemotherapy, surgery and anaesthesia. However, this low incidence is likely to increase due to efficacy of current cancer therapies and prolonged survival. Our case report suggests that patients treated with the radiotherapy for head and neck malignancies should be followed up carefully as early diagnosis and treatment at an early stage is crucial to sarcomas.



