-
PDF
- Split View
-
Views
-
Cite
Cite
N Lilic, MT Mowjood, MHW Wong, A rare and sinister variant of a common ailment: Fungal malignant otitis externa, Journal of Surgical Case Reports, Volume 2012, Issue 9, September 2012, Page 4, https://doi.org/10.1093/jscr/2012.9.4
Close - Share Icon Share
Abstract
A recent case report in this journal highlighted the pathophysiology and management of bacterial malignant otitis externa (MOE) (1). We describe the case of an elderly gentleman who had a delayed diagnosis of fungal MOE with advanced diseased at time of diagnosis. This case highlights the changing microbiology of this serious disease and the difficulty in diagnosis given the rarity of this form of otitis externa relative to its uncomplicated form.
INTRODUCTION
MOE is a rare but well recognised sequelae of otitis externa. Its pathophysiology has been well identified and progresses from otitis externa to a skull base osteomyelitis secondary to pathogens spreading through anatomical planes within and surroudning the temporal bone (2). The most common pathogen identified is Pseudomonas aeruginosa and it is treated with anti-pseudomonal antimicrobial agents unless cultures indicate otherwise.
CASE REPORT
A 70 year old gentleman with a background of type two diabetes mellitus first presented to his family doctor in Februrary of 2011 with left sided, throbbing otalgia that had been present for a week. The doctor examined him and found an oedematous and narrowed external auditory meatus. A swab was taken and clioquinol/flumetasone pivalate ear drops were prescribed for a working diagnosis of otitis externa.
The patient presented one month later with continuing ear pain and a new headache. Examination findings of the left ear were unchanged from first presentation. Framycetin sulphate/gramicidin/dexamethasone drops were prescribed for a diagnosis of ongoing Otitis Externa and analgesia was prescribed for the headache. Over the next three weeks the patient presented on multiple occasions with worsening headache, impaired balance and trismus. Again the clinical findings remained unchanged despite further treatment with oral antiobiotics and further drops. The swab from the first visit had grown Scedosporium apiospermum. Further ear swabs failed to culture any microorganisms.
Two months after his initial presentation, he presented to his family doctor with ongoing otalgia, headache, unsteadiness and had now developed anoxeria. An urgent otorhinolaryngology review found a polyp in the left ear canal with ongoing signs of otitis externa associated with a retained cotton bud. He was prescribed oral and topical ciprofloxacin.
Now three months after his initial presentation, the patient still had ongoing symptoms including headaches, vomiting and 10 kg of weight loss over this time. The headache was atrributed to cervical osteoarthritis, previously diagnosed on CT scanning the year prior. His family doctor admitted him to a small peripheral hospital for management of his pain. At this time a repeat CT scan showed extensive abnormality of the left temporal bone and opacification of the left middle ear, external auditory canal and mastoid air cells. He had a T-tube inserted and was continued on oral and topical Ciprofloxacin for a working diagnosis of mastoiditis secondary to Otitis Externa and media. He was discharged on Methadone for ongoing pain.
Despite the intensification of antimicrobial therapy, over the next few weeks his condition worsened with weakness, headaches, dizziness, falls and otorrhea. He was admitted to a large tertiary hospital for nuclear medicine imaging for suspected MOE.
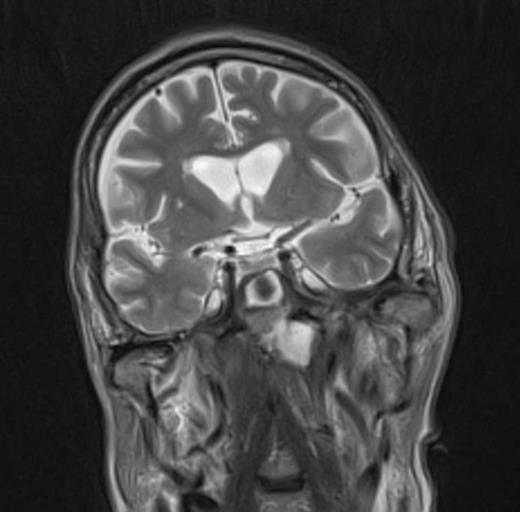
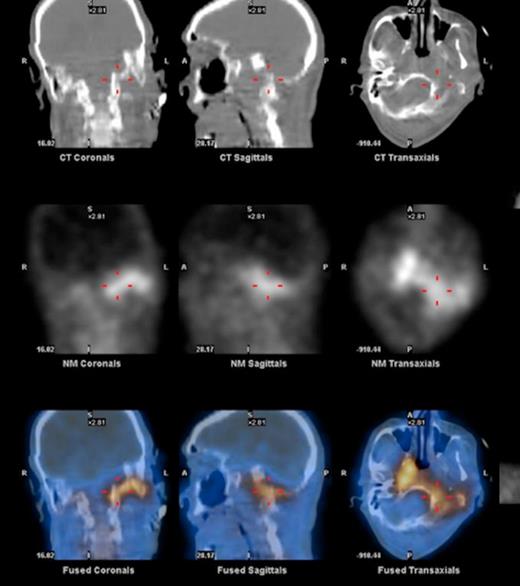
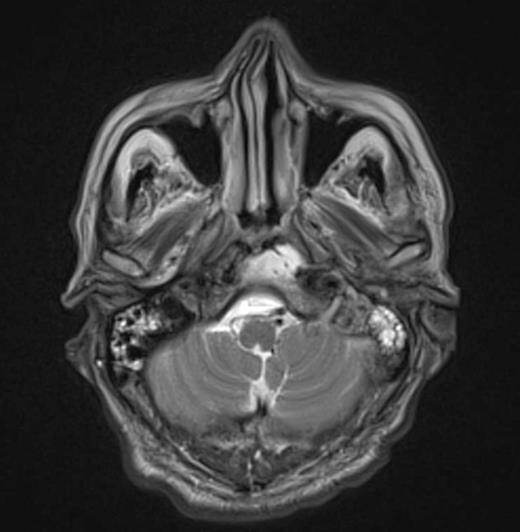
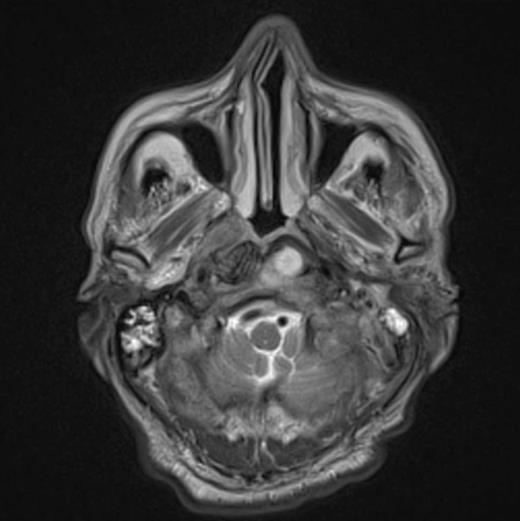
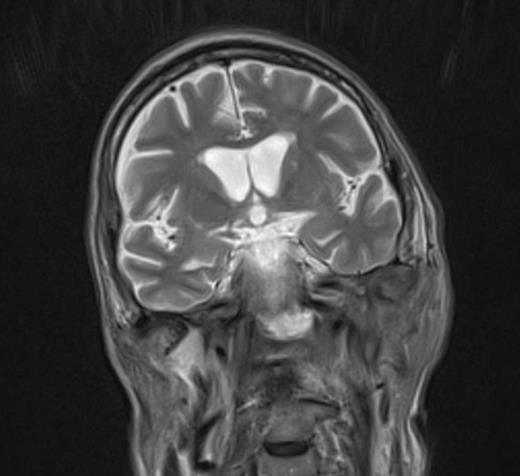
At the tertiary hospital the patient was found to be clinically wasted and weak. He also had rightward uvular deviation, absent gag reflex, weakness of the left sternocleidomastoid and trapezius, House Brackman grade 2 left facial nerve weakness and an immobile left vocal cord. This was the first time a cranial nerve examination was recorded. MRI and SPECT/CT with Gallium tracer was performed, the images acquired from which illustrate the severe extent of disease (figures 1 to 5).
He was diagnosed with MOE with extensive skull base osteomyelitis producing multiple lower cranial neuropathies. Biopsy performed by ORL/ENT surgeons grew Scedosporium apiospermum and given the extent of the infection a palliative approach was agreed upon with the patient and family.
DISCUSSION
The elderly patient described in this case had diabetes mellitus type 2. Advanced age and immunocompromised states including diabetes mellitus are the primary pre-disposing factors to developing MOE (3). Diabetes is an integral aetiological factor for MOE in the elderly and this has been attributed to small vessel disease and impairment of phagocytic function (3).
The vast majority of Otitis Externa is due to bacterial pathogens and so is the case for MOE. The most common pathogen identified is Pseudomonas aeruginosa and up until a decade ago fungal MOE was considered extremely rare (4,5). There are however a number of cases in the literature reporting fungal organisms causing MOE, including Asperigillus species (6-8), Candida parapsilosis (9), Candida ciferri,Pseudoallescheria boydii, Malassezia sympodialis and Scedosporium apiospermum (5,10).
This case adds to the growing evidence that fungal MOE is becoming more prevalent in our community. A possible cause for this is that there is ready access to Ciprofloxacin and other anti-pseudomonal agents in the modern era, therefore reducing the disease burden of Otitis Externa due to Pseudomonas in the community while not addressing the relatively unidentified burden of fungal species. Careful clinical judgement is therefore needed when laboratory culture results reveal unusual non-bacterial species. This is particularly important in patients who have a poor response to anti-pseudomonal agents.
This case also highlights the dangers of attributing headaches in the context of other symptoms to “simple” causes such as cervical osteoarthritis and it is a reminder to always consider an alternative diagnosis to the working diagnosis. Care should always be taken when a patient presents with headaches and systemic upset in the context of otalgia and ear discharge, as the consequences of failing to treat MOE are dire. The mortality rate is currently around 10% with early detection and modern anti-microbials, but if it is identified late MOE can be very difficult to treat due to its propensity to spread. (2)



