-
PDF
- Split View
-
Views
-
Cite
Cite
C O’Meara, T Mahasneh, P Wilson, B I’Ons, D Alkhawaja, Dural prostate metastasis resembling a chronic subdural haematoma, Journal of Surgical Case Reports, Volume 2012, Issue 5, May 2012, Page 7, https://doi.org/10.1093/jscr/2012.5.7
Close - Share Icon Share
Abstract
Subdural hematoma (SDH) is a common neurosurgical pathology, characteristically recognised on plain CT and can be treated with simple and effective surgical intervention. In contrast, dural metastatic adenocarcinoma of the prostate with SDH and malignant extension into the subdural membranes is extremely rare. We describe the case of a 62-year old Caucasian male, provide a brief review of the literature, and explore the potential role of neoangiogenesis and disseminated intravascular coagulopathy in SDH development.
INTRODUCTION
Poorly differentiated metastatic prostate carcinoma to the axial skeleton and visceral organs is common (1), while metastasis to cerebral parenchyma is uncommon (0.63% of cases) and is associated with poor prognosis (2). A recent study found that dural prostate metastasis were more frequent (19.5%) than other cancers (3). Despite this finding however, there is a scarcity of literature describing the presence of chronic subdural haematoma (cSDH) associated with dural metastasis, malignant invasion of the subdural membranes and concurrent DIC.
CASE REPORT
A 62-year old male presented to the emergency department of our institution with epistaxis, anaemia (Hb: 48g/L) and thrombocytopenia (platelets: 52x109/L). Past history included a diagnosis of metastatic prostate adenocarcinoma 7 years earlier. He denied headaches and there were no neurological deficits other than mild cognitive impairment. He was noted to be coagulopathic with an INR of 1.5, despite platelet, fresh frozen plasma and red blood cell transfusion, his coagulopathy ultimately worsened. His level of consciousness deteriorated and a CT Brain was performed identifying acute on chronic SDH (Figure 1 & 2).
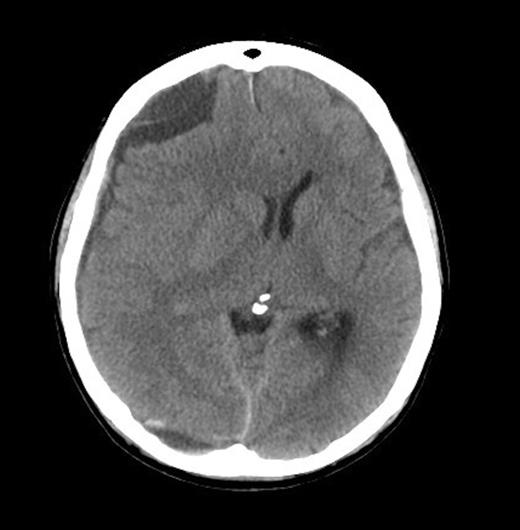
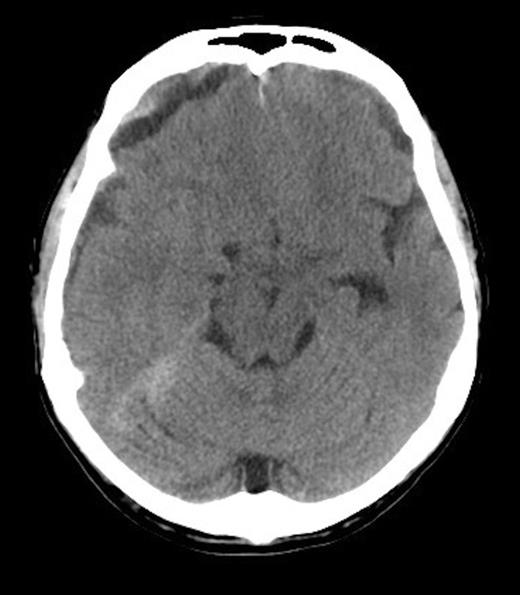
He received further platelet and plasma transfusion to correct his INR of 3.5, and was taken to theatre where a right parietal craniotomy and left parietal burrhole were performed to evacuate the SDH. Intraoperatively, the dura was noted to be thickened and subdural membrane tissue was obtained for histopathology. This was consistent with metastatic adenocarcinoma of the prostate (Figure 3 & 4). The patient recovered well day one post-operatively with no neurological deficits. His level of consciousness rapidly deteriorated on day two post-operatively, with no evidence of haematoma recurrence or neurosurgical complication on repeat CT Brain. The patient subsequently died on post-operative day four.
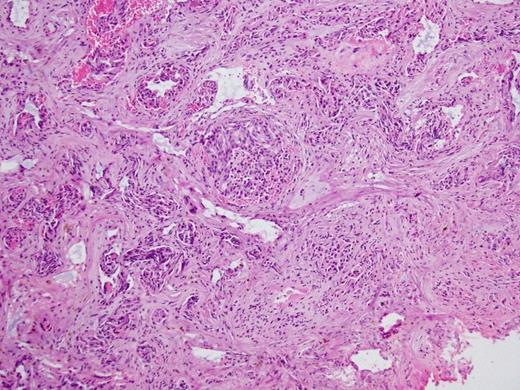
Histopathology Right subdural membranes: extensive infiltration by poorly differentiated adenocarcinoma cells with glandular infiltration (x10).
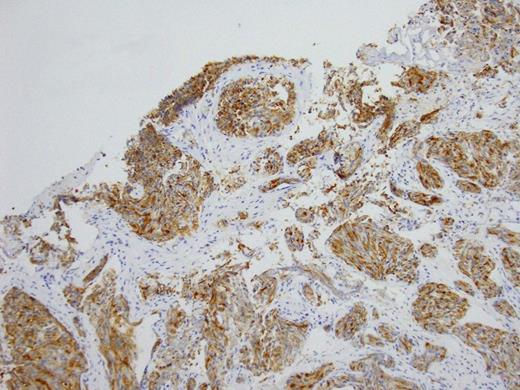
Histopathology Right subdural membranes: Immunoperoxidase staining for PSA (x4)
DISCUSSION
From our review, there have been five reported cases of SDH secondary to metastatic prostate carcinoma to the dura (4-8). Only two of these (including the current case) have shown extension of malignant cells into the subdural membranes.
In the setting of metastatic cancer refractory to therapeutic intervention, it is important to ensure optimal quality of life. Evacuation of mass lesions in the subdural space is a procedure associated with relatively low morbidity and mortality and will improve cognitive ability. Concurrent DIC in our patient may have compromised his prognosis significantly.
A characteristic feature of malignant prostate carcinoma is its ability to promote tissue invasion (through MMP9), cancer cell survival (Caveolin-1 upregulation and promotion of cell survival through Akt-mediated activit and angiogenesis. Endothelial cell dysfunction in tumor microvasculature results in increased vascular permeability (9). In unison, these features enable invasion of the dura, underlying membranes and promote leakiness that is compounded by coagulopathy.
DIC represents the result of a widespread activation of coagulation pathways and is the most frequent coagulation complication in prostate cancer (10). In acute DIC there is a massive generation of thromboplastic material, as well as a consumption of haemostatic elements. Compensatory mechanisms are not sufficient to restore coagulation proteins and platelets. Consequently, transfusion of blood products is often indicated.
It has been postulated that SDH development, in the presence of dural prostate metastasis, might be secondary to dural venous obstruction, hemorrhagic effusion (due to dural metastasis) or an angiodesmoplastic response of the dura to the invasion by carcinomatous cells. We hypothesize that tumor cell invasion of SDH membranes (with permeable microvasculature) in association with coagulopathy (secondary to DIC due to disease progression) may also be potential mechanisms.
In patients with refractory metastatic prostate adenocarcinoma, concurrent DIC and a deteriorating level of consciousness, a differential diagnosis of SDH would enable expedient diagnosis and review by a neurosurgical team.



