-
PDF
- Split View
-
Views
-
Cite
Cite
O Moussa, L Sreedharan, J Poels, T Ojimba, Psoas abscess complicating endovascular aortic aneurysm repair, Journal of Surgical Case Reports, Volume 2012, Issue 10, October 2012, Page 16, https://doi.org/10.1093/jscr/2012.10.16
Close - Share Icon Share
Abstract
Aortic stent graft infection is a rare but serious complication associated with high mortality. This report emphasizes the need for continued awareness of potential graft-related septic complications in patients undergoing Endovascular Aortic Repair (EVAR). We report a case in which a post-EVAR patient became unwell about 30 days post operatively and was shown on CT scanning to have a psoas abscess. The abscess was managed with percutaneous drainage and antibiotics. The patient remains well with no evidence of psoas collection or perigraft infection one year on. We review the available literature and discuss the merits of different management strategies.
INTRODUCTION
Endovascular Aneurysm Repair (EVAR) is now well established as treatment option for abdominal aortic aneurysms (AAA). Up to 70% of all infrarenal AAAs are now treated endovascularly. Like all other surgical procedures that involve the implantation of non-degradable prosthesis, stent-graft infection is a dreaded complication, but it is fortunately rare.
Although there is no convincing evidence, it is generally accepted that the incidence of aortic graft infection is higher with open repair (OR) than with EVAR. Once established infected aortic grafts can lead to bacteraemia, para-aortic abscesses, psoas abscess and aorto-enteric fistulae. Operative intervention is usually required to remove the infected graft. A variety of reconstruction options are available to re-establish arterial flow to the pelvis and lower limbs. About 1.4% of infected aneurysms are complicated by psoas abscess formation (1).
We report a case in which an otherwise uneventful EVAR was complicated a month later by development of a psoas abscess. We discuss our management strategy and review other management options as detailed in the literature.
CASE REPORT
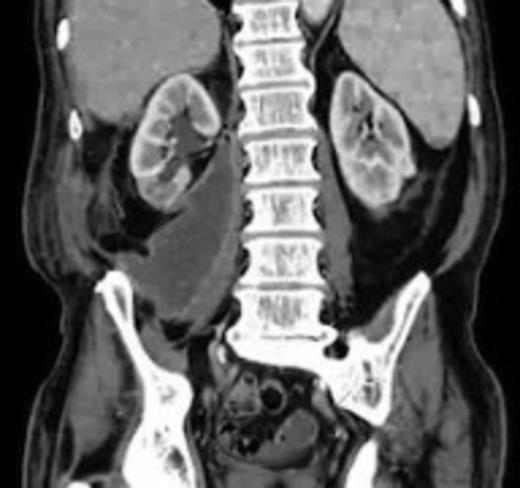
An 82 year old gentleman with known peripheral vascular disease and previous open popliteal aneurysm repair underwent endovascular repair of a 58mm infrarenal abdominal aortic aneurysm (Fig. 1) using a Cook Zenith bifurcated prosthesis The Zenith Flex® AAA Endovascular Graft (Cook Medical Inc., Bloomington, USA). The operation, which was performed under general anaesthetic and bilateral groin cut downs with intravenous prophylactic antibiotic cover on induction (Co-amoxiclav 1.2g) was uneventful. The main body was deployed via the right side. Completion angiograms showed satisfactory exclusion of the aneurysm sac with good graft position and no endoleaks. Post operatively the patient had a brief period of low grade pyrexia and lower abdominal pain that settled spontaneously. This was thought to be due to post implant graft reaction. Pre-discharge imaging with plain abdominal X-rays was deemed satisfactory.
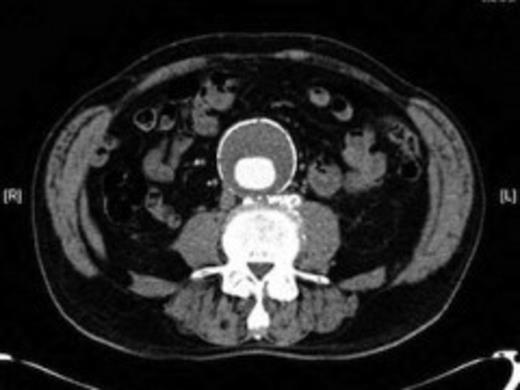
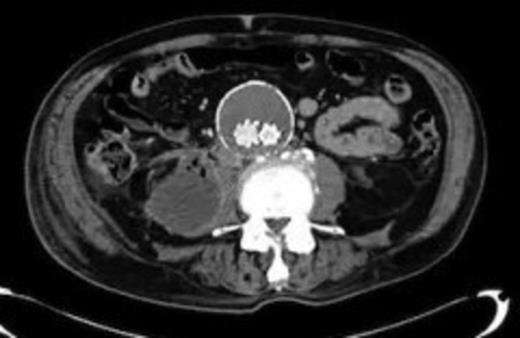
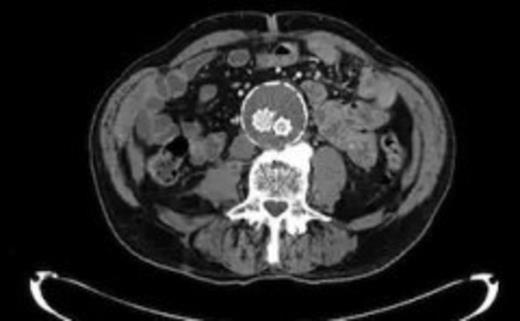
Four weeks following discharge the patient was re-admitted with feeling increasingly unwell, recurrent pyrexia, loss of appetite and mild shortness of breath on exertion. Physical examination was unremarkable except for a temperature of 38.3 centigrade. Blood tests on admission showed Hb 11.4 g/dl (11.5-16.0 g/dl), white cell count 13.1×109/L (4-9.2 x 109/L) and CRP 68mg/L (<3 mg/L). Contrast enhanced CT scan (Fig. 2,3) showed a 50 mm maximum retroperitoneal collection situated between the right kidney and the right iliac vessels. It contained a few loculi of gas. A diagnosis of postoperative right psoas abscess was made. The patient was started on intravenous broad spectrum antibiotics – Tazocin (Piperacillin+ Tazobactam) and Metronidazole. CT guided drainage was carried out using a 10 French gauge pigtail catheter locked pigtail (Meditech Flexima regular all-purpose drainage catheter sets with locking pigtail (Boston Scientific)) (Fig. 4). The isolation of E. coli from the collection with negative Hemocultures suggested a bowel source for the infection but no actual breach was demonstrable. The bowel surgeons ruled out colonic pathology.
A 50 mm maximum retroperitoneal collection situated between the right kidney and the right iliac vessels
A 50 mm maximum retroperitoneal collection situated between the right kidney and the right iliac vessels
The patient's condition improved quite rapidly and the intravenous antibiotics were stopped and replaced with oral ciprofloxacin upon microbiological advice (for a total of six weeks). The drain was removed after 2 weeks when the series of repeat CT scans showed the abscess had nearly completely resolved (Fig. 5). Further CTs on follow up at 6 weeks and 3 months post discharge show no collection at all. At the last clinic visit 6 months following his last admission the patient was enjoying good health.
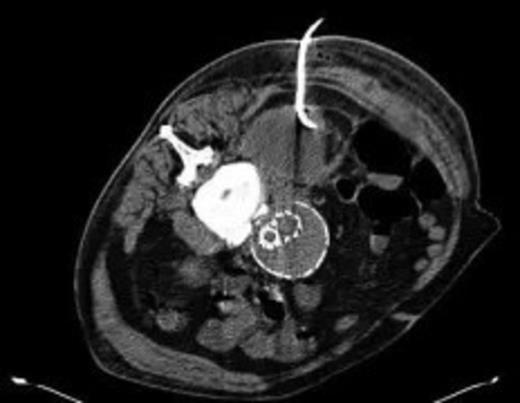
CT guided drainage was carried out using a 10 French gauge pigtail catheter locked pigtail (Meditech Flexima regular all-purpose drainage catheter sets with locking pigtail (Boston Scientific))
Near complete resolution of psoas collection after 2 weeks of intravenous antibiotics
DISCUSSION
Aortic endograft infection (AEI) is fortunately rare, 0.1% according to the Euro registry. This rate is much lower than rates for open abdominal aortic aneurysm repairs (0.4 – 0.7%) (2). Re-interventions, emergency presentation and endoleaks appear to increase the incidence of graft sepsis. It is not known whether mode of access i.e. femoral cut down vs. percutaneous access has any effect on infection rates.
There have been a few reports of psoas abscess collections complicating aortic stent graft placement in the literature. Onset is insidious and diagnosis is difficult. Recurrent fever, general unwellness and haematological signs of sepsis in a post op EVAR patient should arouse suspicion. CT scanning is usually diagnostic but white cell scintigraphy or PET scans may be required. Repeated Blood cultures are often necessary to identify the responsible pathogen. Sharif et al reported 2 cases of psoas abscesses amongst 6 cases of AEI out of 509 EVAR cases over an 8-year period (3). Both required open surgical graft excision and bypass. Hulin and Morris (4) reported a case of left psoas abscess collection that was treated successfully by open surgical drainage, authors attempted first CT guided drainage and conservative treatment that failed, without graft explantation.
Conventional surgical wisdom dictates that any infected prosthetic graft should be excised in-total to eradicate infection (3). This approach was favoured by Sharif et al. Similarly, Laser et al (5) treated 9 cases of AEI all by open surgical explantation with in hospital mortality of 2 cases. This approach often means difficult and extensive surgery in these often very sick patients with generally high morbidity and mortality. A number of authors have therefore advocated graft preservation (3,6,7). Some treat all patients initially with antibiotics and then intervene surgically as appropriate.
We elected to treat our patient with intravenous antibiotics and CT guided drainage because the abscess seemed fairly localised and there was little perigraft collection. In conclusion patients with psoas collections following EVAR can be successfully managed by conservative mean and prolonged antibiotics in selected cases.



