-
PDF
- Split View
-
Views
-
Cite
Cite
Miguel Angel Reyna Silva, Maria Samantha Hernandez González, Rubi Carolina Estrada Hernández, Jazmin Montserrat Guzmán Diaz, Alejandro González Ojeda, Clotilde Fuentes Orozco, Maria F Reyes Ponce, German Quiroga Moreno, Sandra Elisea Plascencia Guerrero, Guadalupe Castillo Cardiel, Gabino Cervantes Guevara, Enrique Cervantes Pérez, High-grade malignant peripheral nerve sheath tumor of the esophagus: a rare case highlighting the diagnostic value of immunohistochemistry, Journal of Surgical Case Reports, Volume 2026, Issue 2, February 2026, rjaf1052, https://doi.org/10.1093/jscr/rjaf1052
Close - Share Icon Share
Abstract
This case report presents a highly unusual case of a primary high-grade malignant peripheral nerve sheath tumor (MPNST) of esophagus, a neoplasm of extreme rarity, with fewer than twenty histologically confirmed cases reported worldwide to date; detailing the successful management of this tumor through Orringer’s transhiatal esophagectomy, complemented by comprehensive histopathologic and immunohistochemical evaluation. Our report emphasizes the crucial the role of immunohistochemistry, specifically the diagnostic value of S100, SOX10, and the exclusion of gastrointestinal stromal tumors markers (DOG1, CD117) in distinguishing MPNST, from morphologically similar esophageal submucosal tumors.
Introduction
Primary malignant tumors of the esophagus rank 11th worldwide among cancer types; soft-tissue sarcomas comprise <1% [1]. MPNSTs (5–10% of sarcomas; lifetime incidence 0.001%) are rare neoplasms arising from Schwann cells or other peripheral nerve sheath components; most occur in the extremities or retroperitoneum, rarely involve esophageal (~19 case, 1993–2025) [2–6].
MPNSTs may occur sporadically, post-radiation, or in association with neurofibromatosis type 1 (NF1), which confers an 8–13% lifetime risk. Most are high-grade tumors expressing S-100 protein; weak or absent expression correlates with poor differentiation and higher metastatic potential. Immunohistochemical loss of H3K27me3 is a sensitive diagnostic marker, observed in 69% of MPNSTs, while tumor side, p53 overexpression, and margin status are key prognostic factors [7].
Due to its rarity, esophageal MPNST poses significant diagnostic challenges, often mimicking leiomyomas or gastrointestinal stromal tumors (GISTs). Diagnosis relies on histopathologic and immunohistochemical correlation. Clinically, progressive dysphagia predominates, with odynophagia, dyspnea, or chest pain occurring less frequently [5]. Given the scarcity of cases, treatment follows general sarcoma surgery principles, and long-term outcomes remain uncertain [3].
Case report
A 48-year-old male presented with a two-year history of progressive dysphagia accompanied by chest pain, without prior radiation exposure, NF1, or other malignancy risk factors. Esophagogastroduodenal contrast series (Fig. 1) revealed a radiolucent, intraluminal mass in the distal esophagus causing partial obstruction. Consequently, an upper endoscopy (Fig. 2) was performed, which confirmed an obstructive tumor in the lower third of the esophagus, with associated duodenitis and erosive gastritis.
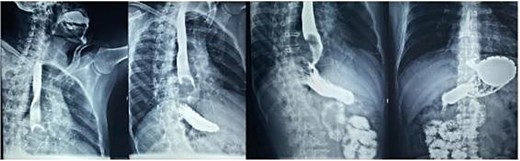
Esophagogastroduodenal series: Radiolucent image in the distal third of the esophagus forming a convex mass due to partial obstruction of the passage of contrast into the stomach.
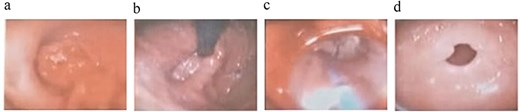
Endoscopic findings: (a) esophageal lesion; (b) hiatal hernia; (c) esophageal dilations; (d) gastric antrum.
Endoscopic biopsies were negative immunostaining for DOG1 and CD117, ruling out a GIST, but positivity for actin with nuclear atypia, suggesting a smooth-muscle tumor of uncertain malignant potential. Contrast-enhanced computed tomography (CT) scan (Fig. 3) showed a 67 × 51 × 47 mm lobulated intraesophageal lesion with central necrosis and strong enhancement, originating from the posterior esophageal wall and nearly occluding the lumen, without regional lymphadenopathy, local invasion, or metastases.
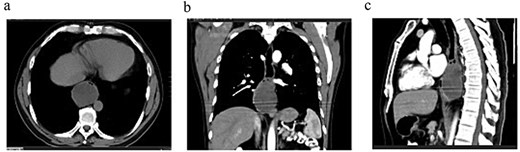
Contrast-enhanced thoracoabdominopelvic CT scan: (a) axial section showing an oval intraesophageal mass; (b) coronal section illustrating the longitudinal extent of the tumor; (c) sagittal section showing a tumor apparently respecting the pericardium.
A transhiatal esophagectomy (Orringer’s technique) with two-layer cervical esophagogastric anastomosis was performed (Fig. 4). Intraoperatively a 7 × 4 cm tumor occupying 99% of the esophageal lumen in the middle and distal thirds was identified. Postoperatively, the patient received mixed enteral and parenteral nutrition and developed an esophagocervical fistula that resolved with conservative management within 20 days.
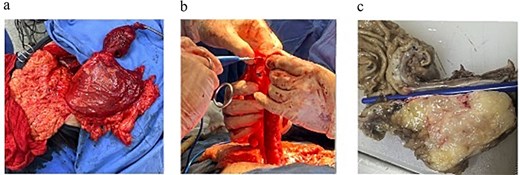
(a) Surgical specimen following transhiatal esophagectomy showing a large esophageal tumor mass. (b) Gastric tube formation for posterior mediastinal ascent. (c) Sectioned specimen displaying a well-defined, lobulated, firm mass.
Histopathological analysis revealed a spindle-cell mesenchymal neoplasm with interlacing fascicles, palisading areas, and low mitotic index (1–2 per 50 high-power fields). Immunohistochemical showed positivity for S100, SOX10, desmin, and myogenin, and a Ki-67 index of 20% (Fig. 5), confirming the diagnosis of a high-grade MPNST.
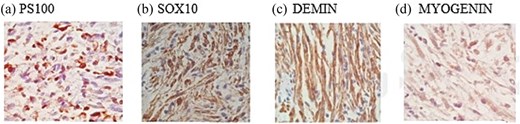
Immunohistochemical staining of the esophageal neoplasm: Positive expression of S100 (a) and SOX10 (b) consistent with neural differentiation, and co-expression of desmin (c) and myogenin (d) indicating muscle differentiation.
Discussion
Dysphagia may have either a neuromuscular or structural origin; in this case, its gradual onset suggested a structural origin. Nakagawa et al. (2023) reviewed 17 reported cases of esophageal MPNST, identifying dysphagia as the most common presenting symptom [3]. CT remains essential for evaluation, as illustrated both in our case and previous reports, showing large intraluminal esophageal masses with heterogeneous enhancement and necrosis [6].
MPNSTs macroscopically resemble submucosal tumors and are usually undiagnosable by routine biopsy, which makes their distinction challenging. In our case, the initial biopsy suggested a smooth-muscle tumor of uncertain malignant potential [3]. Despite the lack of definitive diagnosis, a transhiatal esophagectomy (Orrienger’s technique) was performed, providing complete tumor resection without the need for thoracotomy. This approach, originally designed for achalasia, has been successfully adapted for selected esophageal neoplasms, offering favorable outcomes [1, 8].
Complete radical resection remains the cornerstone of treatment for primary MPNST, as these tumors exhibit aggressive local behavior and potential for metastasis. Although recurrence has not been documented in cases of esophageal resection, thorough preoperative assessment is essential to define the most appropriate surgical plan. The role of adjuvant radiotherapy or chemotherapy remains limited; however, increasing understanding of the molecular signaling pathways involved in MPNST has opened the possibility of targeted therapeutic approaches [3].
Immunohistochemistry is critical for diagnostic confirmation, as it provides definitive identification in a significant proportion of soft-tissue sarcomas [9]. In this case, negative staining for DOG1 and CD117 excluded GIST, while strong S-100 and SOX10 expression, markers highly sensitive for neural-crest-derived cells and strongly indicative of neural tumors, confirmed the neural origin of the tumor [10–13]. In contrast, actin positivity suggested a smooth-muscle tumor, leading to an initial consideration of leiomyosarcoma [14]. Combined with the histopathologic features and a Ki-67 index of 20%, these established the diagnosis of high-grade MPNST.
This case reinforces the importance of a multidisciplinary diagnostic approach integrating imaging, histopathology, and immunohistochemistry to accurately differentiate esophageal MPNST from morphologically similar neoplasms and to guide optimal surgical management in this exceptionally rare entity.
Conflict of interest statement
None declared.
Funding
None declared.
Ethics approval and consent to participate
Not applicable since anonymized patient details and images are used.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.



