-
PDF
- Split View
-
Views
-
Cite
Cite
Alexander Friedman, Dan Eisenberg, Outcomes of revisional bariatric surgery in patient with hypermobile Ehlers–Danlos syndrome, Journal of Surgical Case Reports, Volume 2025, Issue 9, September 2025, rjaf782, https://doi.org/10.1093/jscr/rjaf782
Close - Share Icon Share
Abstract
Ehlers–Danlos syndrome (EDS), a rare group of inherited connective tissue disorders caused by alterations in collagen synthesis, has the potential for disrupted tissue healing leading to morbidity in patients undergoing gastrointestinal surgery. There is a paucity of literature on the safety of metabolic and bariatric surgery (MBS) in individuals with a diagnosis of EDS. We report on the outcomes of a patient with hypermobile subtype EDS who underwent sleeve gastrectomy for management of class III obesity. The patient underwent revisional Roux-en-Y gastric bypass for insufficient weight loss, without increased morbidity. This report suggests that primary (stapled) and revisional (anastomotic) MBS are safe in individuals with hypermobile subtype EDS. However, there are 13 subtypes of EDS, and it is likely that each is associated with different levels of surgical risk. A thorough discussion of potential implications of a diagnosis of EDS should be part of informed consent for MBS.
Introduction
Ehlers–Danlos syndrome (EDS) is a rare heterogeneous group of inherited connective tissue disorders that are caused by alterations in collagen synthesis. Traditionally, they have been characterized by joint hypermobility, skin hyperextensibility, and connective tissue fragility [1, 2]. Consequently, there is concern for altered tissue healing and increased complication risk following surgery in individuals with this diagnosis, especially during operations requiring gastrointestinal (GI) resection and/or anastomosis [3].
Staple-line and anastomotic leak are a significant source of potential morbidity following metabolic and bariatric surgery (MBS), raising concern for increased risk of complication in patients with EDS. It is critical to determine whether patients with a diagnosis of EDS can undergo MBS safely, without complications related to tissue healing. However, there are few reports of primary (or revisional) MBS in this patient population. In this study, we describe a single-institution experience with revisional MBS in a patient with EDS.
Case report
A 40-year-old female with hyperlipidemia, metabolic dysfunction-associated fatty liver disease, lumbar back pain and osteoarthritis presented for treatment of class III obesity (body mass index [BMI] = 49.5 kg/m2). Past medical history was significant for hypermobile-type EDS. She had a history of excessive uterine bleeding, occasional bruising, but no prior surgery, no other history of delayed wound healing. Medications included a 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitor, acetaminophen, nonsteroidal anti-inflammatory drug, and a multivitamin that included vitamin C. Preoperative optimization by a dedicated interdisciplinary team of healthcare providers at an MBS referral center [4] included assessment for modifiable obesity-related comorbidity and baseline activity, and comprehensive laboratory testing that demonstrated normal blood counts and coagulation profile.
Laparoscopic sleeve gastrectomy was performed as primary MBS. Staple line reinforcement with bioabsorbable glycolide copolymer was used, as is standard in our institution. At the conclusion of the operation, a percutaneous drain was positioned along the staple line, which was considered high-risk due to the patient’s diagnosis of EDS. Postoperative course was uneventful, and the patient was monitored in the hospital for 2 days prior to discharge. There were no early or late complications.
After 3 years, the percent excess BMI loss was deemed inadequate (%EBMIL = 27%), and the patient was started on a GLP-1 receptor agonist resulting in modest, but insufficient additional weight loss. In preparation for conversion of sleeve gastrectomy to gastric bypass, the patient was again evaluated by the same dedicated interdisciplinary team.
Robot-assisted conversion of sleeve gastrectomy to Roux-en-Y gastric bypass (RYGB) was performed using our standard 5-port technique. Again, a drain was placed along the gastrojejunostomy. The patient had an uneventful postoperative course with initiation of diet on postoperative day 1 and was discharged home on day 2. Close postoperative follow-up visits were planned after 1 week, 2 weeks, 2 months, 6 months, 12 months, and annually thereafter. At the 6-month visit, the incision sites healed well, the patient continued to tolerate the prescribed diet, and BMI was 36.8 kg/m2 (representing 9.6% additional total weight loss and 24.4% additional excess weight loss since revision to RYGB) (Fig. 1).
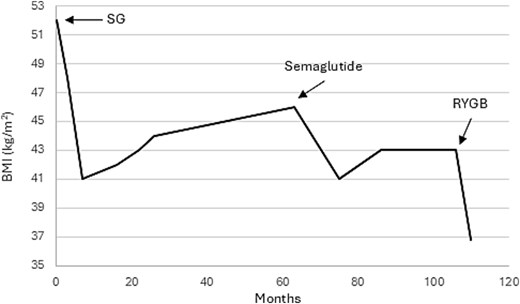
Trajectory of BMI loss over time after sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB).
Discussion
The diagnosis of EDS is rare, but given the high prevalence of obesity, it is anticipated that bariatric surgeons will encounter this diagnosis in their patients. Yet there are few reports of MBS in patients with a diagnosis of EDS. Here, we describe revisional MBS in a patient with hypermobile-type EDS without increased complications and with good weight loss outcomes.
There are 13 subtypes of EDS. The most common are hypermobile EDS (hEDS) with an incidence of approximately 1 in 5000–20 000, the classical EDS (cEDS) and classical-like EDS (clEDS) subtypes with an incidence of approximately 1 in 30 000, and vascular EDS (vEDS), which occurs in an estimated 1 in 100 000 to 200 000 people [1].
Previous studies have highlighted an increased need for emergency general surgery in patients with EDS, as well as surgery-related complications after GI surgery. A systematic review of 11 studies with a total of 1567 patients with EDS found that there were higher rates of spontaneous viscous perforation, most commonly in the sigmoid colon, which were associated with high mortality, particularly in the vEDS subtype [5, 6]. Interestingly, spontaneous ventral hernias occurred in up to 19% of patients with EDS, including rates of 23% in patients with vEDS subtype. Patients with the vascular subtype also experienced higher rates of vascular aneurysm, dissection, and spontaneous rupture, as well as higher post-surgical complications, including postoperative hemorrhage.
Another study of patients with vEDS who underwent elective or emergent colonic surgery were found to have elevated rates of anastomotic leaks [7]. In addition, surgery was associated with overall high postoperative complication rates, including intra-abdominal bleeding, wound dehiscence, and vascular complications.
Our findings in patients undergoing MBS are consistent with those of others. Verdure et al. followed seven patients with EDS who underwent MBS; five patients had hEDS, one had cEDS, and one patient had vEDS. The patient with vEDS experienced excessive postoperative hemorrhage requiring reintervention. The remaining six patients had uneventful postoperative recovery and all seven were doing well at one year follow-up [8]. A study by Herdes et al. reported on the experience of sleeve gastrectomy in two adolescent patients with hEDS and found no postoperative complications [7]. As in our report of a patient undergoing primary and revisional MBS, it remains unclear whether specific measures should be taken to preoperatively optimize patients with EDS prior to MBS. Furthermore, data suggests that surgical risk may vary among different EDS subtypes.
This report suggests that revisional MBS is safe in patients with hEDS, and no specific preoperative optimization, changes in operative technique or postoperative management is required. It is unclear whether this finding is generalizable to other subtypes of EDS and a thorough discussion with the patient of potential implications of a diagnosis of EDS should be part of informed consent.
Conflict of interest statement
None declared.
Funding
None declared.
References
- obesity
- ehlers-danlos syndrome
- informed consent
- weight reduction
- operative risk
- surgical procedures, operative
- diagnosis
- morbidity
- surgery specialty
- connective tissue hereditary disorder
- gastrointestinal surgical procedures
- bariatric surgery
- gastric bypass, roux-en-y
- sleeve gastrectomy, laparoscopic
- collagen synthesis



