-
PDF
- Split View
-
Views
-
Cite
Cite
Maika Yoshioka, Masafumi Shimoda, Kaori Abe, Nanae Masunaga, Masami Tsukabe, Tetsuhiro Yoshinami, Yoshiaki Sota, Tomohiro Miyake, Tomonori Tanei, Kenzo Shimazu, Isolated splenic metastasis presenting at diagnosis of HER2-positive de novo metastatic breast cancer: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 9, September 2025, rjaf751, https://doi.org/10.1093/jscr/rjaf751
Close - Share Icon Share
Abstract
Splenic metastasis is uncommon, and truly isolated splenic metastasis (ISM) from breast cancer is a clinical rarity. Contemporary autopsy series reports splenic involvement in <1% of breast cancer-related deaths, and fewer than 15 well-documented ISM cases have been published. We report a 71-year-old woman presenting with a right-breast mass, regional lymphadenopathy, and a solitary splenic lesion detected on staging computed tomography (CT) and positron emission tomography-CT. Biopsy confirmed hormone receptor-negative, HER2-positive invasive ductal carcinoma. Six cycles of docetaxel, trastuzumab, and pertuzumab led to a 59% reduction in the primary tumour and complete radiological resolution of the splenic lesion. The patient remains progression-free 22 months after initiating therapy, maintained on trastuzumab and pertuzumab. Although extremely rare, ISM can present as the initial manifestation of de novo metastatic breast cancer. Awareness of this possibility may facilitate early systemic therapy and obviate the need for diagnostic splenectomy.
Introduction
Splenic metastasis is considered rare due to unfavourable mechanical factors (tortuous arterial entry, rhythmic contraction) and an immunologically hostile microenvironment. In large autopsy compilations encompassing over 2000 deaths due to solid tumours, splenic metastases were observed in only 3% of all cases and 0.3%–0.7% of breast cancer-related deaths [1–3]. A 25-year pathological survey identified breast cancer as the origin in 12% of splenic secondaries; however, these were typically associated with disseminated disease rather than isolated splenic involvement [4].
True isolated splenic metastasis (ISM) from breast cancer is, therefore, a clinical curiosity. Systematic searches (PubMed through July 2025; Japana Centra Revuo Medicina) yielded fewer than 15 well-documented cases, with only one identified synchronously at the time of breast cancer diagnosis. Here, we report an additional synchronous ISM case and summarize its favourable response to HER2-targeted chemotherapy.
Case report
A 71-year-old woman with a remote history of right-upper-lobectomy for Stage IIIA lung cancer (performed 20 years earlier, with no recurrence) underwent routine surveillance computed tomography (CT) in February 20XX, revealing a mass in the right breast and a low-density lesion in the spleen.
On clinical examination, a 4-cm ill-defined mass was palpated in the right breast, accompanied by a positive dimpling sign. Mammography showed an ill-defined, high-density mass in the upper-outer quadrant. Ultrasound revealed a 34-mm hypoechoic lesion with irregular margins and two swollen subclavian lymph nodes without an echogenic hilum. Dedicated breast MRI showed a 45-mm irregular, enhancing mass with early wash-in/fast wash-out kinetics (BI-RADS category 4C). Contrast-enhanced CT and positron emission tomography-CT identified a right breast mass, enlarged subclavian lymph nodes, and a solitary 2-cm splenic lesion (SUVmax 5.5) without additional distant lesions (Fig. 1A and B).
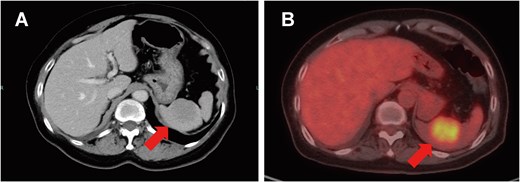
Splenic metastasis. Contrast-enhanced CT (A) and positron emission tomography-CT (B) demonstrate a solitary 2-cm splenic mass (arrows).
Vacuum-assisted biopsy of the right breast mass confirmed histological-grade 3 invasive ductal carcinoma, estrogen receptor-negative, progesterone receptor-negative, and HER2/ERBB2-positive (IHC 3+, FISH ratio 6.04) with a Ki-67 index of 70%. Fine-needle aspiration of a right subclavian lymph node yielded malignant cells consistent with breast carcinoma metastasis. The final pre-treatment diagnosis was right breast cancer, cT2N3aM1, cStage IV, HER2-enriched subtype.
First-line treatment with docetaxel, trastuzumab, and pertuzumab administered every three weeks was initiated in June 20XX. Following six cycles (October 20XX), contrast-enhanced CT demonstrated a 52% reduction in the breast mass and complete resolution of the splenic lesion (Fig. 2). At re-evaluation in April 20XX + 1, the primary mass had shrunk by 59%, and the spleen remained clear. Maintenance dual anti-HER2 therapy is ongoing, and the patient remains progression-free at 22 months.
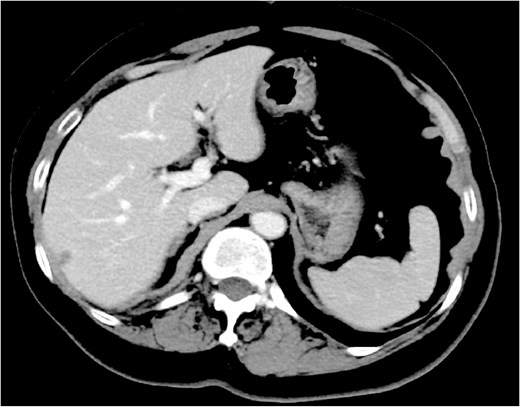
Effect of chemotherapy plus anti-HER2 therapy on splenic metastasis. Contrast-enhanced CT following six cycles of docetaxel, trastuzumab, and pertuzumab shows complete resolution of the splenic lesion.
Discussion
Multiple autopsy surveys consistently report a frequency of splenic metastasis from breast cancer below 2.8%. Schön et al. reviewed 1898 solid-tumor autopsies (1980–1999) and identified splenic metastasis in 57 cases (3% overall); only seven were attributed to breast cancer, representing 0.4% of all autopsied cancers [1]. Nash & Sampson reported a rate of 0.7% of splenic metastasis cases caused by breast cancer (4 of 544 cases) in a series of autopsies in 1966 [2], while Herbert documented 2.8% in an earlier series of 1000 autopsy cases [3]. In one autopsy-based study, half of the patients with splenic metastasis had involvement of five or more organs [5]. Hence, while the spleen may occasionally be affected in disseminated disease, it is seldom a primary metastatic site in breast cancer.
ISM is even less common. Our updated literature review identified fewer than 15 well-documented cases of breast cancer-related ISM (Table 1). Only two—including the present case—were synchronous with the primary diagnosis; the remaining cases occurred 5–240 months after definitive breast surgery. The pathogenesis remains speculative, although hematogenous seeding via the splenic artery is presumed.
| Author (Year) . | Age (Years) . | Interval from primary to ISM . | Receptor status . | Management of the splenic lesion . | Outcome . |
|---|---|---|---|---|---|
| Barreca et al. 2001 [6] | 56 | 24 months post-mastectomy | ER+/HER2− | Splenectomy | Alive, NED (18 months) |
| Iype et al. 2002 [7] | 50 | 8 years post-mastectomy | ER+ | Splenectomy | Alive, NED (12 months) |
| Iga et al. 2009 [8] | 46 | 19 years post-therapy | ER+ | Splenectomy | Alive, NED (6 months) |
| Sufficool et al. 2013 [9] | 63 | 2 years post-mastectomy | ER+/HER2− | Splenectomy | Alive (NR) |
| Hironaka et al. 2014 [10] | 69 | 5 years post-mastectomy | ER+/HER2− | Splenectomy | Alive, NED (8 months) |
| Chen et al. 2022 [11] | 55 | 19 months post-therapy | ER−/HER2− | Splenectomy | Alive (3 months) |
| Dang et al. 2025 [12] | 62 | Synchronous | NR | Systemic therapy | Alive (NR) |
| Present case | 71 | Synchronous | ER−/HER2+ | Systemic therapy | Alive (22 months) |
| Author (Year) . | Age (Years) . | Interval from primary to ISM . | Receptor status . | Management of the splenic lesion . | Outcome . |
|---|---|---|---|---|---|
| Barreca et al. 2001 [6] | 56 | 24 months post-mastectomy | ER+/HER2− | Splenectomy | Alive, NED (18 months) |
| Iype et al. 2002 [7] | 50 | 8 years post-mastectomy | ER+ | Splenectomy | Alive, NED (12 months) |
| Iga et al. 2009 [8] | 46 | 19 years post-therapy | ER+ | Splenectomy | Alive, NED (6 months) |
| Sufficool et al. 2013 [9] | 63 | 2 years post-mastectomy | ER+/HER2− | Splenectomy | Alive (NR) |
| Hironaka et al. 2014 [10] | 69 | 5 years post-mastectomy | ER+/HER2− | Splenectomy | Alive, NED (8 months) |
| Chen et al. 2022 [11] | 55 | 19 months post-therapy | ER−/HER2− | Splenectomy | Alive (3 months) |
| Dang et al. 2025 [12] | 62 | Synchronous | NR | Systemic therapy | Alive (NR) |
| Present case | 71 | Synchronous | ER−/HER2+ | Systemic therapy | Alive (22 months) |
NR, not reported; NED, no evidence of disease.
| Author (Year) . | Age (Years) . | Interval from primary to ISM . | Receptor status . | Management of the splenic lesion . | Outcome . |
|---|---|---|---|---|---|
| Barreca et al. 2001 [6] | 56 | 24 months post-mastectomy | ER+/HER2− | Splenectomy | Alive, NED (18 months) |
| Iype et al. 2002 [7] | 50 | 8 years post-mastectomy | ER+ | Splenectomy | Alive, NED (12 months) |
| Iga et al. 2009 [8] | 46 | 19 years post-therapy | ER+ | Splenectomy | Alive, NED (6 months) |
| Sufficool et al. 2013 [9] | 63 | 2 years post-mastectomy | ER+/HER2− | Splenectomy | Alive (NR) |
| Hironaka et al. 2014 [10] | 69 | 5 years post-mastectomy | ER+/HER2− | Splenectomy | Alive, NED (8 months) |
| Chen et al. 2022 [11] | 55 | 19 months post-therapy | ER−/HER2− | Splenectomy | Alive (3 months) |
| Dang et al. 2025 [12] | 62 | Synchronous | NR | Systemic therapy | Alive (NR) |
| Present case | 71 | Synchronous | ER−/HER2+ | Systemic therapy | Alive (22 months) |
| Author (Year) . | Age (Years) . | Interval from primary to ISM . | Receptor status . | Management of the splenic lesion . | Outcome . |
|---|---|---|---|---|---|
| Barreca et al. 2001 [6] | 56 | 24 months post-mastectomy | ER+/HER2− | Splenectomy | Alive, NED (18 months) |
| Iype et al. 2002 [7] | 50 | 8 years post-mastectomy | ER+ | Splenectomy | Alive, NED (12 months) |
| Iga et al. 2009 [8] | 46 | 19 years post-therapy | ER+ | Splenectomy | Alive, NED (6 months) |
| Sufficool et al. 2013 [9] | 63 | 2 years post-mastectomy | ER+/HER2− | Splenectomy | Alive (NR) |
| Hironaka et al. 2014 [10] | 69 | 5 years post-mastectomy | ER+/HER2− | Splenectomy | Alive, NED (8 months) |
| Chen et al. 2022 [11] | 55 | 19 months post-therapy | ER−/HER2− | Splenectomy | Alive (3 months) |
| Dang et al. 2025 [12] | 62 | Synchronous | NR | Systemic therapy | Alive (NR) |
| Present case | 71 | Synchronous | ER−/HER2+ | Systemic therapy | Alive (22 months) |
NR, not reported; NED, no evidence of disease.
Splenic lesions are readily visualized on CT and positron emission tomography; however, percutaneous biopsy remains underutilized due to concerns about bleeding and tumor seeding. Recent multicenter data show that ultrasound-guided 18-gauge core biopsy is both safe (complication rate < 1%) and diagnostically reliable [13], suggesting it could replace diagnostic splenectomy in many patients.
Given the likelihood of occult systemic disease, current practice favours upfront systemic therapy. Although local approaches such as splenectomy or stereotactic body radiotherapy may be considered in oligometastatic settings within clinical trials (e.g. NRG-BR002, SABR-COMET) [14], no local treatment has yet been proven to improve survival in the metastatic setting. Our patient achieved a durable radiological complete response of the splenic lesion with chemotherapy combined with anti-HER2 therapy, supporting the efficacy of systemic treatment as the primary strategy.
Conclusion
Isolated splenic metastasis is an exceedingly rare presentation of breast cancer (<15 reported cases). Nonetheless, it should be considered in the differential diagnosis of a solitary splenic mass, even during primary staging. Advances in image-guided biopsy and effective systemic therapies enable organ preservation and sustained disease control.
Conflict of interest statement
MS, TY, and KS received honoraria from Chugai. Others have no conflicts of interest to declare.
Funding
This research did not receive any specific grant.
References
- trastuzumab
- computed tomography
- biopsy
- breast cancer metastatic
- hormones
- autopsy
- infiltrating duct carcinoma
- genes, erbb-2
- neoplasm metastasis
- receptor, erbb-2
- splenectomy
- breast
- diagnosis
- neoplasms
- spleen
- breast cancer
- docetaxel
- positron
- systemic therapy
- splenic lesion
- secondary malignant neoplasm of spleen
- her2 positive
- pertuzumab
- lymphadenopathy
- breast cancer death rate



