-
PDF
- Split View
-
Views
-
Cite
Cite
Charlotte Loh, Raghad Alshammasi, Thomas J Crotty, Arooj Fatima, John O’Neill, Dylan J Murray, Colleen B Heffernan, Intraosseous myofibroma of the mandible in a 15 year old: case report and review of the literature, Journal of Surgical Case Reports, Volume 2025, Issue 8, August 2025, rjaf563, https://doi.org/10.1093/jscr/rjaf563
Close - Share Icon Share
Abstract
Myofibromas are rare, benign spindle cell tumours occuring in the first two decades of life, with predilection for the head and neck region. Intraosseous myofibromas of the mandible are exceptionally rare, with fewer than 50 reported cases. We describe the case of a 15-year-old male with an enlarging, painless oral mass causing third molar displacement. Examination revealed an exophytic sublingual lesion with a necrotic surface. Imaging demonstrated a well-defined osteolytic lesion in the mandibular body with soft tissue extension. Biopsy confirmed intraosseous myofibroma, characterized by bland spindle cell proliferation and hemangiopericytoma-like vasculature. The patient underwent a marginal mandibulectomy. Postoperative recovery was uneventful; follow-up imaging at 6 months confirmed no recurrence. Oral rehabilitation is ongoing. This case highlights the importance of accurate diagnosis and multidisciplinary management in treating rare mandibular tumours. Surgical resection remains the definitive treatment.
Introduction
Myofibromas are rare, benign tumours predominantly composed of myofibroblasts—spindle-shaped cells with characteristics of both smooth muscle cells and fibroblasts [1]. Myofibromas may occur as part of a broader condition known as infantile myofibromatosis, a distinct entity characterized by multiple benign tumours, often with systemic involvement [2]. Although most myofibromas arise in soft tissues, intraosseous myofibromas are rare. When these lesions occur, the mandible is the most commonly affected site with fewer than 50 cases reported in the literature in the paediatric population [3–5]. This report presents a rare case of a solitary intraosseous myofibroma of the mandible in a 15-year-old male, contributing to the limited body of literature and reviewing previously reported cases.
Case report
A 15-year-old male presented to the Paediatric Otolaryngology clinic with a progressively enlarging oral cavity mass over a 2-month period. The mass was asymptomatic, aside from intermittent bleeding, which required manual pressure to achieve haemostasis and a loose molar. There was no history of trauma, reduced oral intake, weight loss, or fever. The patient had no significant medical or surgical history or known drug allergies. He denied the use of tobacco, alcohol, or electronic cigarettes.
Clinical assessment revealed a right-sided sublingual swelling extending into the inferior alveolus, displacing the third lower molar tooth. The mass measured ≈ 5 × 6 × 4 cm and exhibited a grey, sloughy region on its superior surface (Fig. 1). There was no active bleeding, tenderness, or purulent discharge. Neck examination showed no swelling or tenderness, and flexible nasendoscopy was normal to the level of the vocal cords.
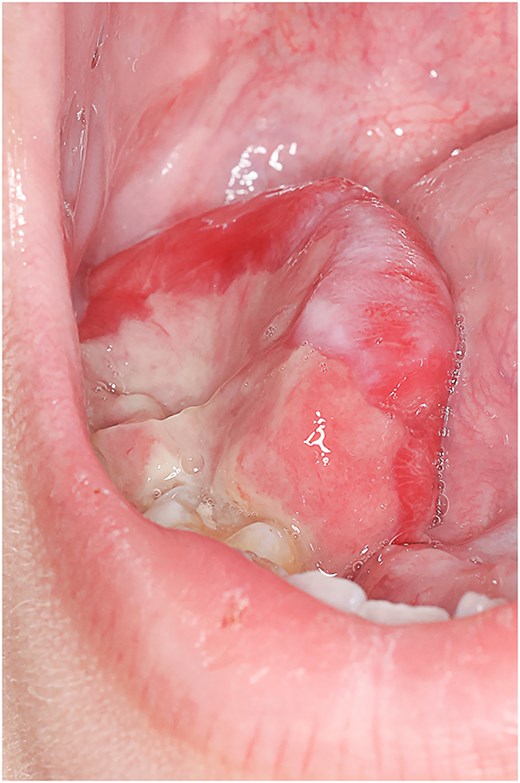
Right-sided sublingual swelling extending into the inferior alveolus, displacing the third lower molar tooth. The mass measured ≈ 5 × 6 × 4 cm.
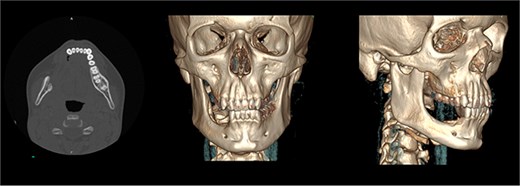
The patient was admitted from the clinic for urgent evaluation. Routine blood investigations were normal. An orthopantomogram revealed displacement of the second and third molars and a poorly defined superior margin of the alveolar process, suggesting osseous involvement (Fig. 2). Contrast-enhanced computed tomography (CT) of the facial bones and neck demonstrated a well-defined lucent lesion within the mandibular body with exophytic soft tissue extension into the oral cavity (Fig. 3). Magnetic resonance imaging (MRI) of the neck illustrated the hyperintense soft-tissue component with no rim enhancement or necrosis (Fig. 4). There was no lymphadenopathy noted clinically or radiograpgically.
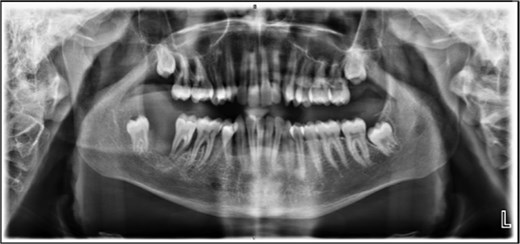
XR OPG showing displacement of the second and third molars and a poorly defined superior margin of the alveolar process, suggesting osseous involvement
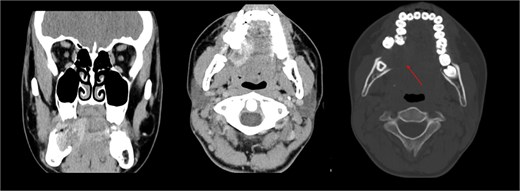
CT showing well defined lucent lesion within the body of the right side of the mandible with exophytic soft tissue component extending into oral cavity.
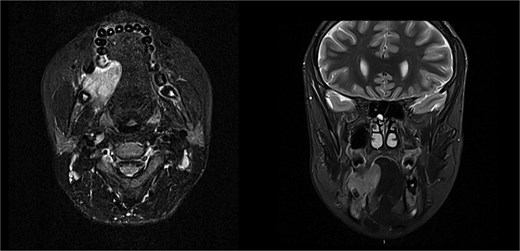
Solid enhancing right osteolytic mandibular lesion with small necrotic components within it. Infection/abscess less likely due to absence of fat stranding/rim enhancement (MRI).
The patient underwent a biopsy of the lesion. Histopathological analysis revealed spindle cell proliferation arranged in a fascicular growth pattern, dilated hemangiopericytoma-like vessels, and a central myeloid nodule. Immunohistochemical staining was positive for smooth muscle actin (SMA), with no evidence of cellular atypia (Figs 5–7).
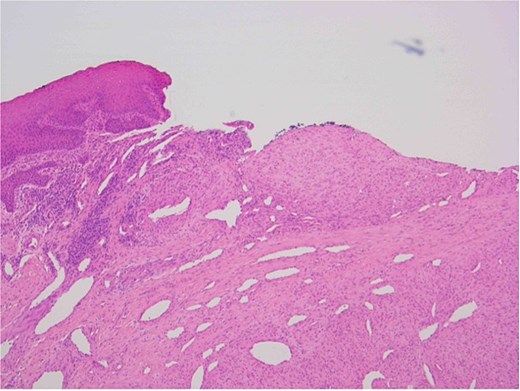
Oral squamous mucosa with underlying bland spindle cell proliferation showing fascicular growth and dilated haemangiopericytoma-like vessels and a myoid nodule centrally.
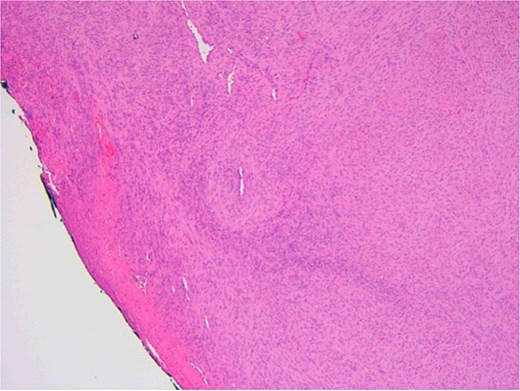
Ulcerated area with underlying bland spindle cell proliferation showing fascicular growth impression of cellularity involving vascular wall – haemangiopericytoma-like architecture (central).
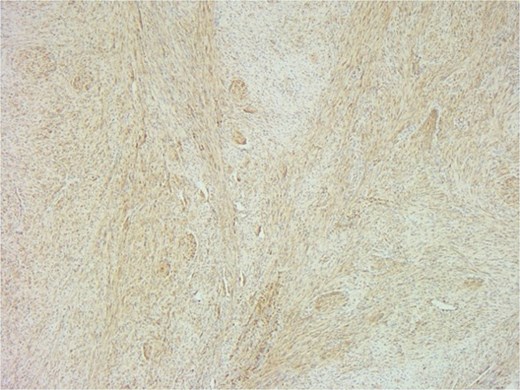
SMA immunostain showing diffuse positivity in spindle cells and highlighting myoid nodules.
The case was reviewed at a multidisciplinary paediatric head and neck tumour meeting, where the diagnosis of intraosseous myofibroma was confirmed. Given the benign pathology, slow growth, and potential for involution, surgical excision was recommended.
Following a thorough discussion, the patient and his parents opted for surgical management. He was referred to the National Paediatric Craniofacial Center and underwent a right-sided marginal mandibulectomy. The tumour was successfully removed with a narrow margin of healthy bone. The residual mandible remained structurally robust and capable of accommodating osseointegrated implants if required in the future.
The postoperative course was uneventful, and the patient was discharged on the first postoperative day. At 3 months postoperatively, follow-up CT imaging (Fig. 8) confirmed no evidence of recurrence. At 6 months, the patient remained symptom-free with normal oral intake and no clinical signs of recurrence.
Discussion
Oral lesions are a common referral to paediatric otolaryngology services. These lesions are typically small and beingn but uncommonly, can present as an aggressive lesion. Intraosseous myofibromas typically present in the first two decades of life, with a mean diagnostic age of 6.7 years and a male predilection [6]. The mandible is a frequent site of involvement within the head and neck region [7, 8]. These tumours are often discovered incidentally due to their asymptomatic nature [4, 6], however they can manifest as palpable swelling due to bony expansion and cortical thinning [4, 6], mobility of teeth is near dental roots as seen in our case and in rare instances, facial asymmetry [5, 6]. The clinical presentation can mimic odontogenic lesions, such as cysts or fibromas, making accurate diagnosis challenging without histopathological examination.
MRI is the optimal modality for diagnosing intraosseous myofibroma of the mandible [9, 10]. MRI provides superior soft tissue contrast and anatomical information, which is crucial for assessing the extent of the lesion and its relationship with adjacent structures. MRI findings include low signal intensity on T1-weighted images and variable signal intensity on T2-weighted images, with peripheral enhancement after gadolinium administration [10]. MRI avoids radiation exposure, which is paramount considering that this tumour commonly affects a younger patient cohort. CT is valuable for evaluating bone involvement and detecting cortical destruction, calcifications, and the extent of bone erosion. OPGs are useful as an initial imaging modality to identify the presence of a mandibular lesion and its effect on teeth.
Histologically, myofibromas show characteristic biphasic pattern with central zone of rounded cells and the peripheral zone of spindle cells [11, 12]. Immunohistochemically, myofibromas are SMA and vimentin positive, and S100 protein and desmin negative, aiding in differentiation from other spindle cell neoplasms [11, 12]. In our case, ameloblastoma was an important differential due to overlapping location and radiographic but aggressive with metastatic potential and high recurrence rates [5]. Thus, immunohistochemical staining is essential for accurate diagnosis [11].
Management of intraosseous myofibroma primarily involves surgical intervention with technique tailored to the lesion's characteristics and the patient’s clinical picture [3, 13]. Close monitoring with regular follow up and imaging may be appropriate for small, non-aggressive, asymptomatic lesions, as myofibromas have the potential for spontaneous involution. The surgical team and patient must accept that a change in the clinical picture will mandate surgical intervention. Less invasive techniques including enucleation and curettage, has demonstrated favourable outcomes with low recurrence rates [3] whilst aggressive tumours may necessitate extensive resection to ensure complete removal and minimize recurrence risk [14], as seen in our case.
Postoperative care, including oral rehabilitation and osseointergrated implant planning [15], is crucial, and optimal management is best delivered through a multidisciplinary team tailored to each patient’s needs.
Conclusion
This case highlights the importance of multidisciplinary collaboration in managing rare paediatric tumours, ensuring precise diagnosis, appropriate intervention, and excellent long-term outcomes.
Conflict of interest statement
The authors have no conflicts of/competing interests to disclose.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
Participant consented to submission of the case report to the journal.
Ethical standards
The authors assert that all procedures contributing to this work comply with the Helsinki Declaration of 1975, as revised in 2008.



