-
PDF
- Split View
-
Views
-
Cite
Cite
Khanh C Pham, Thuy M Le, Retroperitoneal unicentric hyaline vascular variant of Castleman’s disease: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf543, https://doi.org/10.1093/jscr/rjaf543
Close - Share Icon Share
Abstract
Castleman's disease is a rare, benign lymphoproliferative disorder that can occur in various anatomical sites, including the retroperitoneum. Unicentric Castleman disease (UCD), particularly the hyaline vascular variant, often presents as a solitary mass and can mimic malignancy. We reported a case of a 53-year-old man presenting with urinary hesitancy for 3 months. Imaging studies identified a well-defined, hypervascular retroperitoneal mass located anterior to the abdominal aorta and posterior to the pancreas. Endoscopic ultrasound with biopsy was performed, but the results were inconclusive and did not provide the necessary clarity for a definitive diagnosis. Surgical excision was performed, and histopathological examination confirmed a hyaline vascular variant of UCD. This case highlights the diagnostic challenge of retroperitoneal UCD and under-scores the essential role of histopathology in distinguishing it from malignancy. Complete surgical resection is curative, and early recognition can prevent unnecessary delays in management.
Introduction
Castleman's disease (CD) is a rare lymphoproliferative disorder first described by Castleman et al. in 1954 [1]. CD is classified into two main subtypes: unicentric CD (UCD), which involves a single region, and multicentric CD (MCD), which affects multiple regions and may be associated with systemic symptoms or underlying viral infections [1, 2]. Histopathologically, CD can be divided into three variants: hyaline vascular (the most common), plasma cell, and mixed type [3].
The hyaline vascular variant of UCD frequently occurs in the mediastinum but may also present in unusual locations such as the retroperitoneum, pelvis, or hepatic hilum [4, 5]. Retroperitoneal involvement is rare, accounting for ⁓13% of CD cases [5]. UCD is typically asymptomatic and incidentally discovered on imaging studies. Because of its rarity and imaging features similar to other malignancies such as lymphoma, gastrointestinal stromal tumors (GIST), and sarcomas, the preoperative diagnosis of UCD is challenging.
This report describes the case of a retroperitoneal UCD of the hyaline vascular type, emphasizing the diagnostic role of histopathology and the therapeutic role of surgical excision.
Case report
A 53-year-old man without significant past medical or surgical history presented to our center with a 3-month history of urinary hesitancy. He reported no systemic symptoms. Physical examinations revealed no palpable masses or peripheral lymphadenopathy.
Laboratory investigations were within normal limits. Abdominal ultrasound was unremarkable. A contrast-enhanced computed tomography (CT) scan of the abdomen revealed a well-circumscribed, homogeneously enhancing, hypervascular soft tissue mass, located in the retroperitoneum, anterior to the aorta and posterior to the pancreatic head (Fig. 1). The mass contained punctate calcifications and there was no evidence of enlarged lymph nodes.
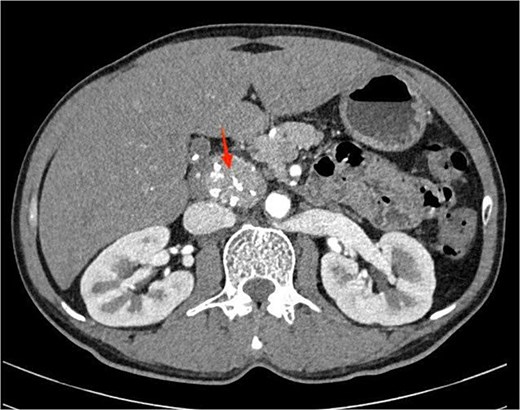
Contrast-enhanced abdominal computed tomography showing a well-defined, homogeneously enhancing retroperitoneal mass with punctate calcifications, located anterior to the aorta and posterior to the pancreatic head.
To further characterize the lesion, endoscopic ultrasound (EUS) was performed, which revealed a hypoechoic mass with clear margins, internal calcifications, and abundant vascularity (Fig. 2). A fine needle biopsy (FNB) using a 22G needle yielded multiple lymphoid cells, but the sample was insufficient for a definitive diagnosis. Given the inconclusive biopsy and persistent clinical suspicion of malignancy, surgical excision was planned.
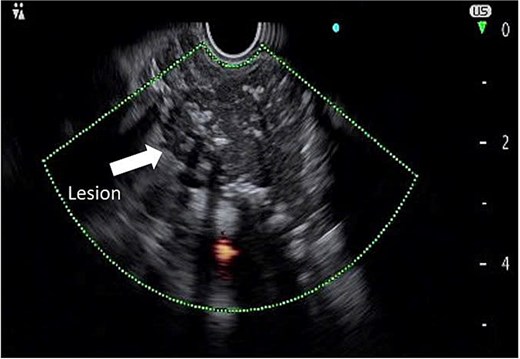
Endoscopic ultrasound image demonstrating a hypoechoic mass with defined margins, internal calcifications, and increased vascular flow.
An exploratory surgery was performed, during which a well-encapsulated retroperitoneal mass was identified near the pancreas. The mass was completely removed with preservation of surrounding structures (Fig. 3). Gross examination showed a 60 × 45 × 35 mm yellow–gray encapsulated mass with scattered calcified foci (Fig. 4).
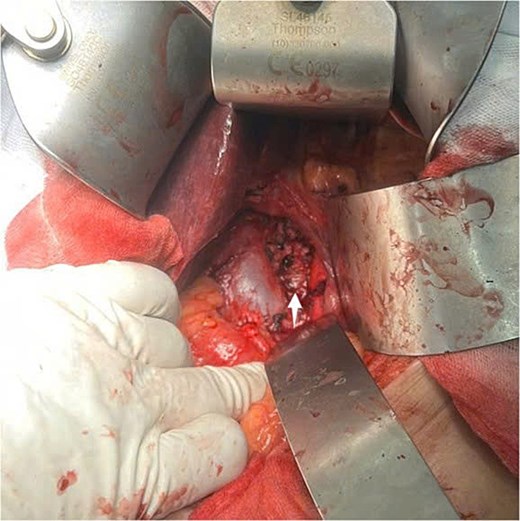
Intraoperative view of the retroperitoneal mass after complete surgical excision (indicated by arrow).
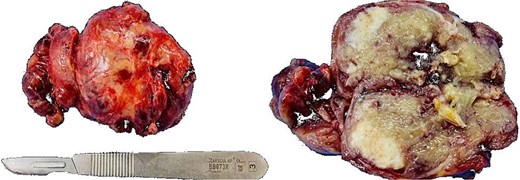
Gross specimen showing a 60 × 45 × 35 mm encapsulated, yellow–gray mass with scattered calcifications.
Histopathological analysis revealed enlarged lymphoid follicles with an ``onion-skin'' appearance, characterized by mantle zone lymphocytes surrounding the atretic follicles (Fig. 5A). Blood vessels penetrate the germinal center, creating a ``lollipop'' appearance for the follicle (Fig. 5B). Additionally, large areas of fibrosis with calcium deposition were observed in some parts of the lymph nodes, and no malignant cells were present. These results were in line with the hyaline vascular variant of CD. The patient recovered uneventfully and was discharged on the fifth postoperative day. At the 1-year follow-up, he remained asymptomatic with no evidence of recurrence.
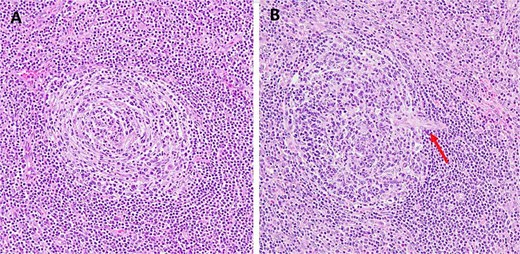
Example of hematoxylin and eosin histologic changes seen in unicentric hyaline vascular Castleman’s disease. (A) The atrophic germinal center surrounded by concentric layers of mantle zone lymphocytes forming the characteristic “onion-skin” appearance (original magnification ×200). (B) Blood vessels (arrow) penetrate the germinal center, creating a ``lollipop'' appearance for the follicle (original magnification ×200).
Discussion
This case emphasizes the diagnostic challenges of retroperitoneal UCD, particularly the hyaline vascular variant. This disease often mimics other retroperitoneal tumors on imaging and remains undiagnosed until surgical excision and histopathologic evaluation.
CD is a rare lymphoproliferative disorder with two main clinical forms: UCD and MCD. The hyaline vascular variant accounts for ⁓80%–90% of UCD cases and is considered benign, with a favorable prognosis following complete surgical resection [1]. In clinical practice, preoperative diagnosis can be quite challenging due to the non-specific imaging features. Contrast-enhanced CT typically shows a well-circumscribed, homogeneous, hypervascular mass with internal calcifications, as seen in this case. These findings are consistent with the hyaline vascular variant of UCD, as described by Kun et al. in their case series study [6].
Endoscopic ultrasound (EUS) is useful in evaluating retroperitoneal and peripancreatic neoplasms. Typically, EUS can help distinguish CD from lymphoma. Lesions that are hypoechoic, homogeneous, with clear boundaries and rich blood flow are more likely to be related to UCD [7]. Additionally, UCD tends to have a softer consistency and more abundant blood supply compared to lymphoma [7]. Fine needle biopsy (FNB) presents an opportunity to obtain pathological tissue for histopathological analysis, potentially allowing for a preoperative pathological diagnosis, as seen in the case by Khashab et al. [8]. However, the yield of FNB remains controversial due to low specificity and increased risk of bleeding [9]. In our case, the EUS-guided biopsy was non-diagnostic, consistent with previous reports [10, 11].
Histopathological confirmation remains the cornerstone of diagnosis. The characteristic findings of the hyaline vascular subtype include small, regressed germinal centers surrounded by concentric layers of mantle zone lymphocytes, and vessels penetrating into the follicles, forming the classic “onion-skin” and “lollipop” appearances, respectively [3]. These features were present in our patient’s resected specimen and confirmed the diagnosis.
Due to its benign progression and minimal invasion of surrounding structures, laparoscopic surgery for UCD is the preferred choice [12]. However, open surgery may be required when the lesion is closely associated with major vascular structures, as in our patient. Long-term follow-up of UCD is recommended. It is recommended that patients after surgery have a follow-up visit each year for the first 3 years and again at 5 years postoperatively for routine CT scans [13].
Several similar cases have been documented. Singh et al. reported a case of retroperitoneal UCD that mimicked a GIST and was diagnosed only after resection [9]. Wang et al. analyzed 14 cases of retroperitoneal UCD and emphasized the importance of complete resection and long-term surveillance [12]. Shi et al. also described a peripancreatic mass that required histopathological confirmation after failed preoperative diagnosis [11]. Our case contributes to this increasing literature and emphasizes the importance of histopathology for accurate diagnosis in challenging presentations.
Conclusion
Retroperitoneal UCD, especially the hyaline vascular variant, is a rare and often unsuspected diagnosis due to nonspecific clinical presentation and radiologic similarity to malignant tumors. This case highlights the critical role of histopathological examination in establishing an accurate diagnosis and guiding appropriate treatment. Complete surgical excision remains the mainstay of therapy and is associated with an excellent prognosis.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
No financial support was provided for the study.
Patient consent
Informed consent was obtained from the patient.



