-
PDF
- Split View
-
Views
-
Cite
Cite
Hwan Il Kim, Kyoung Won Yoon, Hyo Jin Kim, Daun Jeong, Small bowel ischaemia—a rare complication of diabetic ketoacidosis: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf498, https://doi.org/10.1093/jscr/rjaf498
Close - Share Icon Share
Abstract
Small bowel ischaemia, though rare in diabetic ketoacidosis, is a life-threatening condition marked by severe abdominal pain disproportionate to physical findings, potentially progressing to peritonitis and shock if untreated. A 54-year-old male with type 1 diabetes and alcohol dependence presented to the emergency department with persistent abdominal and back pain. He had severe metabolic acidosis, hyperglycaemia, and an elevated anion gap, consistent with diabetic ketoacidosis. A computed tomography scan showed thickening and decreased enhancement of the distal small bowel and ascending colon walls, indicating small bowel ischaemia. Treatment involved restoring blood supply to the ischaemic bowel and surgical resection of necrotic tissue. The patient stabilized postsurgery and was weaned from vasopressors and extubated 2 days later. Renal replacement therapy ceased after 8 days, and he eventually returned to normal life. This case emphasizes the importance of investigating persistent severe abdominal pain in patients with diabetic ketoacidosis to identify critical underlying conditions.
Introduction
Diabetic ketoacidosis (DKA) is a complication of diabetes mellitus (DM) characterized by hyperglycaemia, ketosis, and metabolic acidosis from insulin deficiency. DKA incidence is increasing globally, necessitating improved management strategies. Despite improved diabetes care, DKA remains a significant cause of morbidity and mortality, accounting for 14% of diabetes-related hospital admissions [1].
DKA presents with polyuria, polydipsia, and abdominal discomfort. Abdominal pain, occurring in 40%–75% of cases, can mimic an acute abdomen, causing diagnostic challenges. While abdominal pain typically resolves with metabolic correction, 35% of patients have underlying causes like pancreatitis, hepatitis, or mesenteric ischaemia.
Small bowel ischaemia (SBI) is a life-threatening complication of DKA. It can progress to peritonitis and septic shock without prompt treatment. Diagnosis is challenging due to overlapping symptoms with DKA.
This case report describes DKA-associated SBI, highlighting the importance of early recognition. It includes a literature review of DKA-associated SBI to improve clinical awareness and outcomes.
Case report
A 54-year-old male with type 1 DM and alcohol dependence presented to the emergency department with abdominal pain and blurred vision. After 1 year of alcohol abstinence, the patient consumed 114 g ethanol daily for 1 week and discontinued prescribed insulin injections 4 days before admission.
His vital signs were as follows: blood pressure 164/93 mmHg, heart rate 92/minute, respiratory rate 18/minute, temperature 35.1°C, and oxygen saturation 100% on room air. The patient was alert without neurological deficits. His Glasgow Coma Scale score was E4–V5–M6.
The laboratory results were consistent with DKA. The patient exhibited severe metabolic acidosis with an arterial blood pH of 6.74, exacerbated by hyperglycaemia with a glucose level of 649 mg/dl and a high anion gap of 48.6 mmol/l (Table 1). Lactate was 7.43 mmol/l and serum ketone level was 7565 μmol/l. Tests revealed hyponatraemia, hyperkalaemia, elevated liver enzymes, impaired renal function, leukocytosis, and high triglycerides (Table 2).
| Measurement . | Result . | Reference range . |
|---|---|---|
| pH | 6.739 | 7.350–7.450 |
| PCO2 (mmHg) | 33 | 35.0–48.0 |
| PO2 (mmHg) | 52.5 | 83.0–108 |
| HCO3 (mmol/l) | 4.4 | 21.0–28.0 |
| Total CO₂ (mmol/l) | 5.4 | 22.0–29.0 |
| Base excess (mmol/l) | −31.4 | −2.0 to 3.0 |
| O2 saturation (%) | 76 | 94.0–98.0 |
| Measurement . | Result . | Reference range . |
|---|---|---|
| pH | 6.739 | 7.350–7.450 |
| PCO2 (mmHg) | 33 | 35.0–48.0 |
| PO2 (mmHg) | 52.5 | 83.0–108 |
| HCO3 (mmol/l) | 4.4 | 21.0–28.0 |
| Total CO₂ (mmol/l) | 5.4 | 22.0–29.0 |
| Base excess (mmol/l) | −31.4 | −2.0 to 3.0 |
| O2 saturation (%) | 76 | 94.0–98.0 |
| Measurement . | Result . | Reference range . |
|---|---|---|
| pH | 6.739 | 7.350–7.450 |
| PCO2 (mmHg) | 33 | 35.0–48.0 |
| PO2 (mmHg) | 52.5 | 83.0–108 |
| HCO3 (mmol/l) | 4.4 | 21.0–28.0 |
| Total CO₂ (mmol/l) | 5.4 | 22.0–29.0 |
| Base excess (mmol/l) | −31.4 | −2.0 to 3.0 |
| O2 saturation (%) | 76 | 94.0–98.0 |
| Measurement . | Result . | Reference range . |
|---|---|---|
| pH | 6.739 | 7.350–7.450 |
| PCO2 (mmHg) | 33 | 35.0–48.0 |
| PO2 (mmHg) | 52.5 | 83.0–108 |
| HCO3 (mmol/l) | 4.4 | 21.0–28.0 |
| Total CO₂ (mmol/l) | 5.4 | 22.0–29.0 |
| Base excess (mmol/l) | −31.4 | −2.0 to 3.0 |
| O2 saturation (%) | 76 | 94.0–98.0 |
| Measurement . | Result . | Reference range . |
|---|---|---|
| WBC (103/μl) | 29.42 | 3.5–9.5 |
| Haemoglobin (g/dl) | 14.3 | 13–16.5 |
| Platelet (103/μl) | 293 | 140–400 |
| Total protein (g/dl) | 6.4 | 6.4–8.3 |
| Albumin (g/dl) | 4.2 | 3.5–5.2 |
| Glucose (mg/dl) | 649 | 70–99 |
| Urea nitrogen (mg/dl) | 41 | 6.5–20.9 |
| Creatinine (mg/dl) | 2.24 | 0.70–1.20 |
| eGFR (ml/minute/1.73 m2) | 32 | >60 |
| Total bilirubin (mg/dl) | 1.2 | 0–1.2 |
| Direct bilirubin (mg/dl) | 1.1 | 0–0.3 |
| AST (IU/l) | 2066 | 0–40 |
| ALT (IU/l) | 343 | 0–41 |
| ALP (IU/l) | 173 | 40–129 |
| γ-GTP (IU/l) | 288 | 0–60 |
| LDH (IU/l) | 1879 | 0–225 |
| Amylase (IU/l) | 80 | 13–112 |
| Lipase (IU/l) | 20 | 16–60 |
| CK (mg/dl) | 1757 | 0–190 |
| Uric acid (mg/dl) | 11.7 | 3.4–7.0 |
| Sodium (mEq/l) | 121 | 136–145 |
| Potassium (mEq/l) | 5.8 | 3.5–5.1 |
| Chloride (mEq/l) | 68 | 98–107 |
| Total CO2 (mmol/l) | 3.7 | 22.0–29.0 |
| Calcium (mg/dl) | 8.5 | 8.6–10.0 |
| Magnesium (mg/dl) | 3.1 | 1.9–2.8 |
| Phosphorus (mg/dl) | 15.9 | 2.7–4.7 |
| Triglycerides (mg/dl) | 1880 | 0–150 |
| Total cholesterol (mg/dl) | 224 | 0–200 |
| HDL-cholesterol (mg/dl) | 47 | >60 |
| LDL-cholesterol (mg/dl) | 31 | 0–100 |
| Phospholipid (mg/dl) | 434 | 159–267 |
| Total lipid (mg/dl) | 2650 | 404–814 |
| CRP (mg/l) | 3.2 | 0–5.0 |
| Lactic acid (mmol/l) | 17.43 | 0.36–1.39 |
| Ketone body (μmol/l) | 7565 | 28–120 |
| Measurement . | Result . | Reference range . |
|---|---|---|
| WBC (103/μl) | 29.42 | 3.5–9.5 |
| Haemoglobin (g/dl) | 14.3 | 13–16.5 |
| Platelet (103/μl) | 293 | 140–400 |
| Total protein (g/dl) | 6.4 | 6.4–8.3 |
| Albumin (g/dl) | 4.2 | 3.5–5.2 |
| Glucose (mg/dl) | 649 | 70–99 |
| Urea nitrogen (mg/dl) | 41 | 6.5–20.9 |
| Creatinine (mg/dl) | 2.24 | 0.70–1.20 |
| eGFR (ml/minute/1.73 m2) | 32 | >60 |
| Total bilirubin (mg/dl) | 1.2 | 0–1.2 |
| Direct bilirubin (mg/dl) | 1.1 | 0–0.3 |
| AST (IU/l) | 2066 | 0–40 |
| ALT (IU/l) | 343 | 0–41 |
| ALP (IU/l) | 173 | 40–129 |
| γ-GTP (IU/l) | 288 | 0–60 |
| LDH (IU/l) | 1879 | 0–225 |
| Amylase (IU/l) | 80 | 13–112 |
| Lipase (IU/l) | 20 | 16–60 |
| CK (mg/dl) | 1757 | 0–190 |
| Uric acid (mg/dl) | 11.7 | 3.4–7.0 |
| Sodium (mEq/l) | 121 | 136–145 |
| Potassium (mEq/l) | 5.8 | 3.5–5.1 |
| Chloride (mEq/l) | 68 | 98–107 |
| Total CO2 (mmol/l) | 3.7 | 22.0–29.0 |
| Calcium (mg/dl) | 8.5 | 8.6–10.0 |
| Magnesium (mg/dl) | 3.1 | 1.9–2.8 |
| Phosphorus (mg/dl) | 15.9 | 2.7–4.7 |
| Triglycerides (mg/dl) | 1880 | 0–150 |
| Total cholesterol (mg/dl) | 224 | 0–200 |
| HDL-cholesterol (mg/dl) | 47 | >60 |
| LDL-cholesterol (mg/dl) | 31 | 0–100 |
| Phospholipid (mg/dl) | 434 | 159–267 |
| Total lipid (mg/dl) | 2650 | 404–814 |
| CRP (mg/l) | 3.2 | 0–5.0 |
| Lactic acid (mmol/l) | 17.43 | 0.36–1.39 |
| Ketone body (μmol/l) | 7565 | 28–120 |
Abbreviations: WBC, white blood cell count; eGFR, estimated glomerular filtration rate; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GTP, gamma-glutamyl transferase; LDH, lactate dehydrogenase; CK, creatine kinase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CRP, C-reactive protein.
| Measurement . | Result . | Reference range . |
|---|---|---|
| WBC (103/μl) | 29.42 | 3.5–9.5 |
| Haemoglobin (g/dl) | 14.3 | 13–16.5 |
| Platelet (103/μl) | 293 | 140–400 |
| Total protein (g/dl) | 6.4 | 6.4–8.3 |
| Albumin (g/dl) | 4.2 | 3.5–5.2 |
| Glucose (mg/dl) | 649 | 70–99 |
| Urea nitrogen (mg/dl) | 41 | 6.5–20.9 |
| Creatinine (mg/dl) | 2.24 | 0.70–1.20 |
| eGFR (ml/minute/1.73 m2) | 32 | >60 |
| Total bilirubin (mg/dl) | 1.2 | 0–1.2 |
| Direct bilirubin (mg/dl) | 1.1 | 0–0.3 |
| AST (IU/l) | 2066 | 0–40 |
| ALT (IU/l) | 343 | 0–41 |
| ALP (IU/l) | 173 | 40–129 |
| γ-GTP (IU/l) | 288 | 0–60 |
| LDH (IU/l) | 1879 | 0–225 |
| Amylase (IU/l) | 80 | 13–112 |
| Lipase (IU/l) | 20 | 16–60 |
| CK (mg/dl) | 1757 | 0–190 |
| Uric acid (mg/dl) | 11.7 | 3.4–7.0 |
| Sodium (mEq/l) | 121 | 136–145 |
| Potassium (mEq/l) | 5.8 | 3.5–5.1 |
| Chloride (mEq/l) | 68 | 98–107 |
| Total CO2 (mmol/l) | 3.7 | 22.0–29.0 |
| Calcium (mg/dl) | 8.5 | 8.6–10.0 |
| Magnesium (mg/dl) | 3.1 | 1.9–2.8 |
| Phosphorus (mg/dl) | 15.9 | 2.7–4.7 |
| Triglycerides (mg/dl) | 1880 | 0–150 |
| Total cholesterol (mg/dl) | 224 | 0–200 |
| HDL-cholesterol (mg/dl) | 47 | >60 |
| LDL-cholesterol (mg/dl) | 31 | 0–100 |
| Phospholipid (mg/dl) | 434 | 159–267 |
| Total lipid (mg/dl) | 2650 | 404–814 |
| CRP (mg/l) | 3.2 | 0–5.0 |
| Lactic acid (mmol/l) | 17.43 | 0.36–1.39 |
| Ketone body (μmol/l) | 7565 | 28–120 |
| Measurement . | Result . | Reference range . |
|---|---|---|
| WBC (103/μl) | 29.42 | 3.5–9.5 |
| Haemoglobin (g/dl) | 14.3 | 13–16.5 |
| Platelet (103/μl) | 293 | 140–400 |
| Total protein (g/dl) | 6.4 | 6.4–8.3 |
| Albumin (g/dl) | 4.2 | 3.5–5.2 |
| Glucose (mg/dl) | 649 | 70–99 |
| Urea nitrogen (mg/dl) | 41 | 6.5–20.9 |
| Creatinine (mg/dl) | 2.24 | 0.70–1.20 |
| eGFR (ml/minute/1.73 m2) | 32 | >60 |
| Total bilirubin (mg/dl) | 1.2 | 0–1.2 |
| Direct bilirubin (mg/dl) | 1.1 | 0–0.3 |
| AST (IU/l) | 2066 | 0–40 |
| ALT (IU/l) | 343 | 0–41 |
| ALP (IU/l) | 173 | 40–129 |
| γ-GTP (IU/l) | 288 | 0–60 |
| LDH (IU/l) | 1879 | 0–225 |
| Amylase (IU/l) | 80 | 13–112 |
| Lipase (IU/l) | 20 | 16–60 |
| CK (mg/dl) | 1757 | 0–190 |
| Uric acid (mg/dl) | 11.7 | 3.4–7.0 |
| Sodium (mEq/l) | 121 | 136–145 |
| Potassium (mEq/l) | 5.8 | 3.5–5.1 |
| Chloride (mEq/l) | 68 | 98–107 |
| Total CO2 (mmol/l) | 3.7 | 22.0–29.0 |
| Calcium (mg/dl) | 8.5 | 8.6–10.0 |
| Magnesium (mg/dl) | 3.1 | 1.9–2.8 |
| Phosphorus (mg/dl) | 15.9 | 2.7–4.7 |
| Triglycerides (mg/dl) | 1880 | 0–150 |
| Total cholesterol (mg/dl) | 224 | 0–200 |
| HDL-cholesterol (mg/dl) | 47 | >60 |
| LDL-cholesterol (mg/dl) | 31 | 0–100 |
| Phospholipid (mg/dl) | 434 | 159–267 |
| Total lipid (mg/dl) | 2650 | 404–814 |
| CRP (mg/l) | 3.2 | 0–5.0 |
| Lactic acid (mmol/l) | 17.43 | 0.36–1.39 |
| Ketone body (μmol/l) | 7565 | 28–120 |
Abbreviations: WBC, white blood cell count; eGFR, estimated glomerular filtration rate; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GTP, gamma-glutamyl transferase; LDH, lactate dehydrogenase; CK, creatine kinase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CRP, C-reactive protein.
Noncontrast abdominopelvic computed tomography (CT) showed multifocal air foci within the liver and mesenteric vessels, suggesting possible bowel ischaemia (Fig. 1). After surgical consultation, medical management was recommended as DKA was considered the primary cause. The patient was admitted to the intensive care unit (ICU) for DKA management.
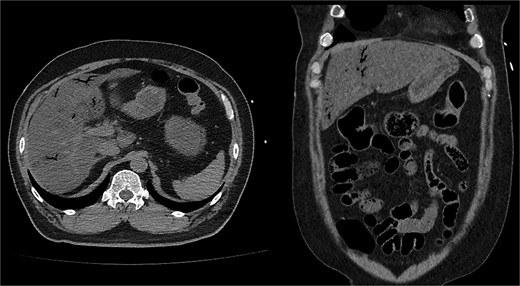
Noncontrast abdominopelvic CT revealed multifocal air foci in the liver and mesenteric vessel, suggesting portal vein and mesenteric vein gas.
On the first day of ICU admission, the patient received 2000 ml crystalloid fluid and 1300 ml bicarbonate mix fluid. Total fluid input was 5583 ml, and output was 720 ml. Blood glucose was managed by continuous insulin infusion with intermittent bolus administration. The patient was treated with ceftriaxone and metronidazole for suspected bowel ischaemia. ABGA revealed the following: pH 7.36, PCO₂ 18.7 mmHg, PO₂ 85.9 mmHg, HCO₃− 10.4 mmol/l, lactate 6.9 mmol/l, and glucose 423 mg/dl. As hourly urine output remained below 0.5 ml/kg, continuous renal replacement therapy (CRRT) was initiated to resolve volume overload. Dynamic liver CT confirmed diffuse fatty liver, significant thickening, and decreased enhancement of walls in the distal SB and ascending colon, supporting SBI diagnosis (Fig. 2).
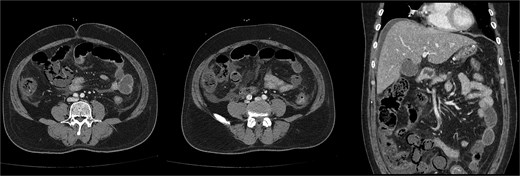
Follow-up dynamic liver CT revealed decreased wall enhancement, mural air densities in the distal small bowel and ascending colon compared to the previous noncontrast abdominopelvic CT.
Due to severe bowel ischaemia, he underwent emergency surgery on the second day, including right hemicolectomy and SB resection, resulting in double-barrel ileocolostomy. The resected and remaining portions of SB measured ~200 cm (Fig. 3).
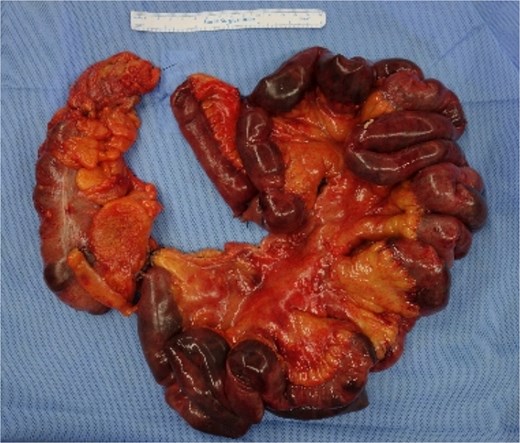
Postoperatively, treatment included broad-spectrum antibiotics (vancomycin, levofloxacin, and meropenem), antifungal agents (micafungin), vasopressors, hydrocortisone, and thiamine to stabilize the patient's condition.
The patient's haemodynamic status improved, leading to vasopressor weaning within 2 days. He was extubated 2 days after surgery, and CRRT ended 8 days after initiation. He transferred to the general ward on Day 15 post-ICU admission. Three months later, he underwent successful colostomy repair and returned to daily life.
Discussion
DKA occurs more frequently in paediatric than adult patients and is strongly associated with type 1 DM but can occur in type 2 DM [2]. The prevalence of DKA in adults varies globally based on diabetes type [3]. The annual incidence of DKA among adult patients with type 1 DM is 5%–8% [4]. This case highlights the need to investigate patients with DKA with persistent severe abdominal pain after initial management to rule out critical conditions.
Abdominal pain, a common nonspecific symptom of DKA, occurs in 40%–75% of patients [5]. The pathophysiology is multifactorial. Gastrointestinal manifestations in DKA result from acute hyperglycaemia-induced impaired gastric motility, leading to atony, ileus, hepatic capsule expansion, and mesenteric ischaemia from volume depletion [6]. Abdominal pain correlates with metabolic acidosis severity [7]. Elevated blood hydrogen ions may stimulate gastrointestinal nerve endings, causing mucosal damage and pain [8]. While DKA usually resolves with metabolic correction after fluid and insulin treatment, persistent severe abdominal pain requires evaluation for conditions needing surgical intervention [9].
SBI, a rare DKA complication, poses clinical challenges due to rapid progression and high mortality. It occurs from severe dehydration, hypotension, and metabolic acidosis causing mesenteric hypoperfusion. DM creates a hypercoagulable state with elevated prothrombotic factors [10].
Both DKA and SBI present with severe abdominal pain, metabolic acidosis, and elevated lactate [11]. No specific markers exist for rapid diagnosis of SBI. SBI shows leukocytosis, hyperphosphataemia, hyperkalaemia, and elevated enzyme levels. While lactic acidosis is common in DKA, elevated lactate may indicate sepsis or SBI, requiring careful clinical interpretation.
In our patient, multifocal air foci within the liver and mesenteric vessels on imaging raised concerns of SBI, confirmed by enhanced CT. Early imaging is crucial for identifying patients at risk for ischaemic complications. Where CT scans are unavailable, chest radiography showing pneumomediastinum, abdominal radiography demonstrating air-fluid levels, and Rigler's sign can provide diagnostic clues for perforation and ischaemia [12]. Despite limitations, chest and abdominal radiography remain accessible tools in resource-limited settings to detect bowel perforation and obstruction from mesenteric ischaemia. However, computed tomography angiography remains the first-line diagnostic modality for assessing intervention necessity [13].
Managing SBI in DKA requires a multidisciplinary approach, combining surgical management with medical intervention. The strategy involves restoring blood supply to the ischaemic bowel and surgical resection of necrotic tissue [14]. Our patient underwent right hemicolectomy and SB resection, with postoperative care including antibiotics, antifungals, vasopressors, and CRRT. This integrated approach is essential for stabilizing patients and preventing complications.
This case emphasizes the awareness of managing DKA-associated SBI that requires systematic diagnosis and intervention.
Acknowledgements
The authors thank the patient and his family for providing informed consent for publication.
Conflict of interest statement
None declared.
Funding
None declared.



