-
PDF
- Split View
-
Views
-
Cite
Cite
Thaddaeus Jun Kiat Tan, Allen Wei-Jiat Wong, Hui Wen Chua, Traction-assisted chest elevation (TRACE)—a novel technique for improving intraoperative ergonomics in endoscopic mastectomy, Journal of Surgical Case Reports, Volume 2025, Issue 6, June 2025, rjaf321, https://doi.org/10.1093/jscr/rjaf321
Close - Share Icon Share
Abstract
Endoscopic mastectomy has provided patients and surgeons with more favorable cosmetic outcomes as compared to standard mastectomy. A major limitation is the increased operative time attributable to technical challenges faced by surgeons. One such challenge faced by procedurists pertains to optimizing the view of the medial breast parenchyma after dissection past the vertex of the breast. We report a technique termed Traction-Assisted Chest Elevation (TRACE) which involves placement of two tension sutures to apply anterior traction at the medial skin flap to optimize endoscopic views for this aspect of endoscopic mastectomy. The optimized ergonomics and improved visualization allows more precise dissection and shortens operative time. Use of TRACE resulted in a reduction of the operative time from an average of 133 min to 90 min. TRACE is a novel technique for optimizing the intraoperative endoscopic view during endoscopic mastectomy and can potentially reduce overall operative time.
Introduction
Minimally invasive breast surgery has introduced new challenges to the surgical management of breast cancer. Endoscopic mastectomy (EM) in particular allows placement of shorter incisions well away from the breast parenchyma which optimizes cosmetic outcomes [1–3], also allowing a range of procedures including reconstruction and augmentation to be performed adjunct to oncologic resection via a single incision [4, 5].
One argument against the routine use of endoscopic mastectomy pertains to increased operative time that is attributable to greater technical difficulty and the longer time required for parenchymal dissection from a smaller incision placed further from the breast parenchyma [6, 7]. One technical challenge is dissecting the skin flap beyond the vertex of the breast via a typical axillary incision. At this point, the surgical endoscope needs to be advanced past the vertex to visualize the medial skin flap which often leads to suboptimal views and a blind spot inferior to the field of vision (Fig. 1A). This can ultimately complicate the dissection across the medial lipocutaneous flap and predispose to operative complications and positive resection margins. To our knowledge, no published studies have described specific means to overcome this challenge.
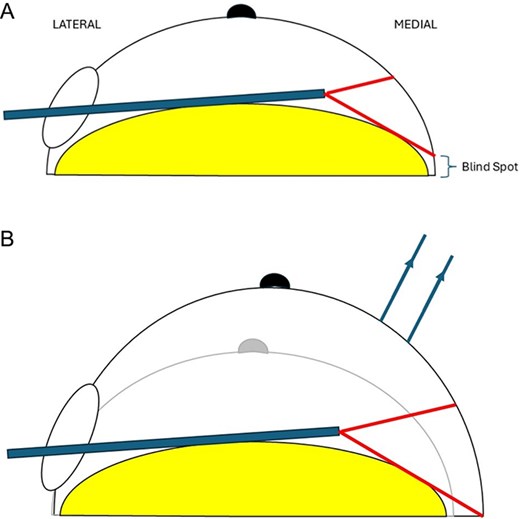
Schematic demonstrating optimization of endoscopic view of medial breast with TRACE technique.
In this study, we describe our experience with applying anterior traction at the medial skin flap as a simple technique that can optimize these views. We describe a technique termed Traction-Assisted Chest Elevation (TRACE).
Surgical technique
We describe a case of a 43-year-old female with cup size C breasts who underwent an endoscopic nipple sparing mastectomy and sentinel lymph node biopsy for biopsy-proven invasive ductal carcinoma of the right breast, and prophylactic nipple sparing mastectomy of the left breast. Our institution’s technique for endoscopic mastectomy has been previously described [8]. A 4 cm mid-axillary incision is made. Sentinel lymph node biopsy is performed for the right breast in the usual technique using dual tracer localization with patent blue dye and indocyanine green. Endoscopic mastectomy is then performed via the same mid-axillary incision. Tumescent fluid (1:1 000 000 adrenaline) is injected in the subcutaneous layer to define the dissection plane. Dissection of the subcutaneous skin flap is done by sharp dissection up to the vertex of the breast. The breast is then taken off the pectoralis major posteriorly with the aid of a lighted retractor and operative rigid endoscope. The dissection is then taken anteriorly to raise the skin flap off the mastectomy specimen.
To optimize visualization past the vertex of the breast, tension sutures are applied to the medial skin flap. This is done with two prolene 2/0 stitches anchored to the medial skin flap to apply traction anteriorly after dissection moves medially past the vertex of the breast parenchyma (Fig. 1). This is termed as TRACE technique. Mastectomy was performed for the contralateral breast in a similar fashion.
In this case, the use of TRACE brings the medial dissection plane into the operative view, as demonstrated in Fig. 2A–D. This obliterates the blind spot at the most medial aspect of this plane. The optimized ergonomics and improved visualization allows dissection to be carried out in a more precise manner. The recorded operative time for this patient was 90 min skin-to-skin for the right breast, 85 min for the left breast, shorter in comparison to the average operative time of 133 min in the same month for cases of endoscopic nipple sparing mastectomy for whom TRACE was not used. The rest of the operative procedure was uncomplicated and postoperative course was uneventful. Final histology revealed a pT2pN0 invasive ductal carcinoma, ER-positive PR-positive and HER2-positive with clear margins on the right. There was no evidence of malignancy on the left breast.
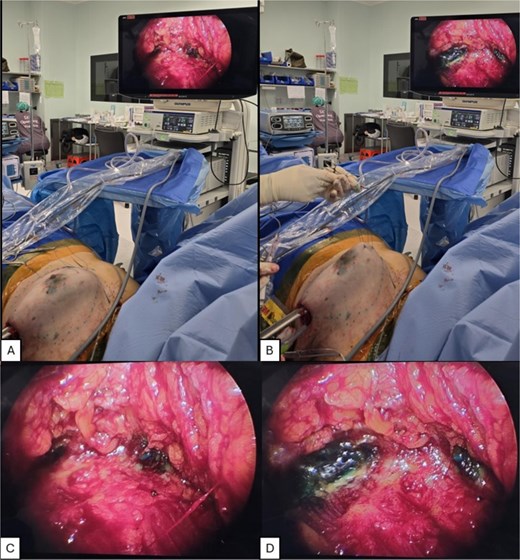
Demonstration of TRACE intraoperative views. (A,C) Without TRACE. (B, D) With TRACE.
Discussion
The impact of endoscopic techniques on oncologic outcomes has been a subject of debate in recent years. Several studies raise concerns regarding its oncologic safety, some suggesting reduced access may render patients at higher risk of positive margins and increased rates of locoregional recurrence [9, 10]. TRACE is a simple and easily reproducible technique that optimizes intraoperative visualization during the dissection of the medial lipocutaneous plane during endoscopic mastectomy. A key strength of the technique is that it does not require any additional equipment and is simple to replicate. Moreover, the technique potentially shortens operative time for endoscopic mastectomy. Nipple-sparing mastectomy for larger volume breasts is known to be very challenging. This technique can alleviate some of that difficulty in a simple and economical fashion.
Recent evidence suggests intraoperative ergonomics and visuals can also be improved with robotic-assisted techniques. Robotic-assisted endoscopic mastectomy was first described by Toesca et al. [11] and several subsequent studies have suggested comparable clinical and oncologic outcomes in comparison with standard endoscopic techniques with the added benefit of improved visualization with 3D optics and better articulation afforded by robotic instruments [12–14]. The limited endoscopic view described in this study may thus be less relevant using a robotic platform, although the increased costs involved may limit the widespread implementation of robotic-assisted minimally invasive techniques.
This study has several limitations. Firstly, as a noncomparative study describing a single patient, no conclusions can be drawn regarding the impact of TRACE on operative time and surgeon ergonomics, and comparative studies with matched controls are required to establish any correlation. Secondly, short- and long-term outcomes at postoperative follow up is not described in this study, and further longitudinal studies would be useful to determine the long-term impact of such a technique on local recurrence rates, surgical margins. It remains nonetheless a pragmatic and simple technique to adopt.
Conclusion
TRACE is a novel technique for optimizing the intraoperative endoscopic view of the medial dissection bed during endoscopic mastectomy. Application of this technique is simple and requires virtually no additional equipment. More studies can be performed to evaluate the effectiveness of this technique in various approaches to endoscopic mastectomy.
Conflict of interest statement
None of the authors have any conflicts of interest to declare.
Funding
This study is not supported by any grants from funding agencies in the public, commercial or non-profit sectors.



