-
PDF
- Split View
-
Views
-
Cite
Cite
Neda Salami, Sharmila Raju, Mehnaaz Mohammed, So Un Kim, Harpreet Gill, Sean Hormozian, Aldin Malkoc, Gladys Vargas, Ahmad Ibrahim, Brandon Woodward, Incidence of pyloric gland adenoma located within the gallbladder, Journal of Surgical Case Reports, Volume 2025, Issue 12, December 2025, rjaf976, https://doi.org/10.1093/jscr/rjaf976
Close - Share Icon Share
Abstract
Pyloric gland adenoma (PGA) of the gallbladder is a rare benign epithelial tumor with potential for malignant transformation. PGAs represent the most common histologic subtype of gallbladder adenomas and are frequently associated with chronic inflammation. Although often incidental findings, their malignant potential warrants careful histopathologic evaluation and consideration for surveillance or surgical management. We present the case of a 64-year-old man with a 30-year history of episodic epigastric pain radiating to the right abdomen and back. Abdominal ultrasound revealed a stalked lesion communicating with the gallbladder, confirmed as a PGA on final pathology after robotic-assisted laparoscopic cholecystectomy.
Introduction
Pyloric gland adenoma (PGA) of the gallbladder is a benign epithelial neoplasm that can arise in the setting of chronic inflammation, such as chronic cholecystitis [1]. These non-invasive lesions are well demarcated from the surrounding mucosa and are composed of pyloric glands arranged in tubular structures [8]. Chronic inflammation can induce metaplastic changes in the gallbladder epithelium and lamina propria, leading to the formation of pyloric-type glandular structures resembling those found in the gastric antrum [2].
Grossly, PGAs appear as soft, tan, thin-stalked lesions that detach easily from the gallbladder surface on pathological inspection and can be mistaken for biliary debris or sludge. Histologically, they have lobular architecture lined by cuboidal or columnar cells. Immunohistochemically, they characteristically express MUC6, a glycoprotein-producing gene associated with mucosal protection of the gastrointestinal tract [2, 3].
Albores-Saavedra et al. [8] reported PGAs as the most common subtype of gallbladder adenoma, occurring predominantly in adult women, usually measuring < 2 cm and presenting as solitary lesions. Gallbladder preinvasive neoplastic lesions, termed intracholecystic papillary neoplasms (ICPN), have four subtypes: gastric, intestinal, biliary, and oncocytic. The gastric subtype (gICPN) and PGAs share morphologic and histologic similarities. While the adenoma–carcinoma sequence is well characterized in colorectal neoplasia, its presence in gallbladder adenomas remains controversial. Like colonic adenomas, gallbladder adenomas may display papillary or tubulopapillary growth patterns, with the tubular form being most common [1].
Case report
A 64-year-old man with rheumatoid arthritis, hypertension, tobacco use, and a history of methamphetamine use presented with a 30-year history of monthly episodes of epigastric pain radiating to the right abdomen and back. Episodes lasted 1 to 2 days, were associated with nausea, vomiting, and night sweats, and resolved after emesis. Pain was unrelated to meals. He reported a previous diagnosis of ‘gallbladder pain’ and attributed his symptoms to this.
An abdominal ultrasound (Fig. 1), obtained before his first clinic visit, showed echogenic densities with shadowing in the gallbladder wall consistent with cholelithiasis. Review of the imaging revealed a stalked lesion communicating with the gallbladder, later confirmed as a PGA. In clinic, he was counseled that his pain was atypical for biliary colic and that a cholecystectomy might not resolve his symptoms. Nonetheless, because the pain was debilitating and impacted his ability to work, he opted for an elective cholecystectomy.
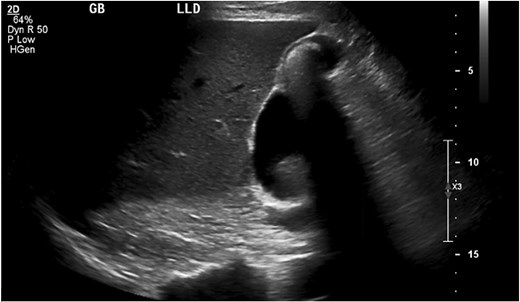
Ultrasound of gallbladder with the pyloric gland adenoma in view.
A robotic-assisted laparoscopic cholecystectomy was performed. Intraoperatively, dense omental and hepatic adhesions overlying the gallbladder complicated exposure. The gallbladder was removed in a standard fashion and sent for pathological analysis. Final pathology revealed chronic cholecystitis, cholelithiasis, and a pyloric gland adenoma. Gross examination showed a single 3.3 cm yellow–brown, crystalline ovoid gallstone, dark green velvety-to-trabeculated mucosa, and a solitary 0.2 cm polyp in the gallbladder body. The histology of the tissue specimen was reviewed by pathology which demonstrated a polypoid lesion with tightly packed pyloric type glands, consistent with PGA histopathology (Figs 2–4).
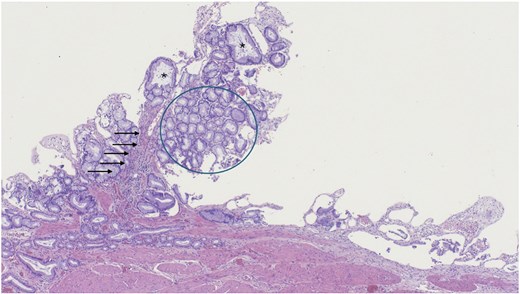
Low magnification of polypoid lesion demonstrating a fibrovascular core (arrows), and tightly packed pyloric type glands (circle) with occasional cystic dilatation (asterisk). Haematoxylin and eosin 4×.
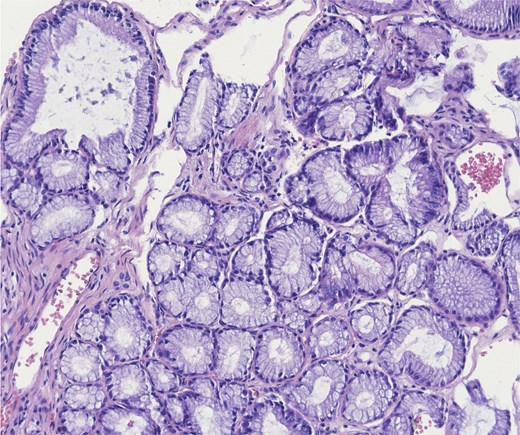
Higher power of bland pyloric glands lined by cuboidal or columnar mucus secreting cells with apical mucinous cytoplasm. Haematoxylin and eosin 20×.
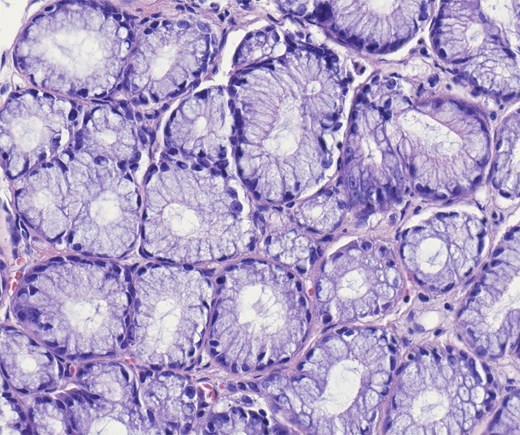
Higher power of bland pyloric glands lined by cuboidal or columnar mucus secreting cells with apical mucinous cytoplasm. No nuclear overlap and no mitosis. Haematoxylin and eosin 40×.
At his 2-week postoperative follow-up, the patient reported complete resolution of his prior abdominal pain, nausea, vomiting, and night sweats. He was tolerating his diet, had normal bowel function, and was referred to surgical oncology and gastroenterology for further evaluation given the malignant potential of PGA.
Discussion
Gallbladder adenomas are rare benign epithelial tumors with malignant potential. They are often asymptomatic and detected incidentally on imaging or after cholecystectomy. Lesions >1 cm are more likely to be symptomatic and carry a higher risk of malignancy [7]. In this surgical case presented, given the small size of the pyloric adenoma, 0.2 cm, it is likely the patient’s presenting symptoms were related to the presence of the ovoid gallstone, with the pyloric adenoma being an incidental finding identified on pathology review.
Histologic and immunohistochemical subtypes include pyloric, intestinal, foveolar, and biliary types, each with differing rates of dysplasia and malignant transformation. Gallbladder adenomas represent ~1.7% of polypoid lesions in cholecystectomy specimens [4, 5]. Pyloric-type adenomas, the most common subtype (up to 82% of cases), have a 27% risk of high-grade dysplasia or carcinoma in situ, though invasive carcinoma is rare. They typically show diffuse MUC6 expression with focal MUC2, MUC5AC, and CDX2 positivity. Intestinal-type adenomas (14%) more often express CDX2 and MUC2 and carry a higher dysplasia/carcinoma in situ rate (46%), though few progress to invasion. Foveolar-type (2.4%) and biliary-type (1.4%) adenomas rarely progress to adenocarcinoma [4, 6].
The adenoma–carcinoma pathway in gallbladder cancer has been linked to p53 and K-RAS mutations. Both pyloric and intestinal types exhibit CTNNB1 and KRAS mutations, though genetic data remains limited [4], and further research is warranted to explore these genetic associations.
Conclusion
This case describes a small (0.2 cm) PGA in a patient with decades-long episodic abdominal pain. The molecular biology, clinical behavior, and malignant potential of gallbladder PGAs remain poorly characterized, and no standardized diagnostic or surveillance guidelines exist. Further research is needed to identify prognostic biomarkers and optimal management strategies.
Conflict of interest statement
The authors declare there is no conflict of interest.
Funding
The research presented in this manuscript had no specific funding from any agency in the public, commercial or not-for-profit sectors.
Informed consent
Informed consent was obtained for use of de-identified information for publication per our Institutional Review Board policy.



