-
PDF
- Split View
-
Views
-
Cite
Cite
Clare B Maher, Brad Guo, Anthony A Dunlop, Inflammatory optic neuritis and scleritis following commencement of semaglutide: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 11, November 2025, rjaf937, https://doi.org/10.1093/jscr/rjaf937
Close - Share Icon Share
Abstract
Given the documented increased risk of non-arteritic ischaemic optic neuropathy (NAION), the widespread use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has raised concerns regarding their ophthalmic safety. This study presents a unique case of inflammatory optic neuritis and scleritis in a 43-year-old female one month following commencement of semaglutide. Extensive investigations for underlying systemic causes, including autoimmune and connective tissue disorders were negative. Treatment with systemic corticosteroids led to a gradual resolution of symptoms and return of vision to baseline over a three-month period. This case highlights a potential link between semaglutide and inflammatory ocular conditions. Clinicians should maintain an open mind when evaluating optic neuropathies in the context of GLP-1 RA use and consider corticosteroid-responsive inflammatory aetiologies as alternatives to NAION. Multicentre population-based cohort studies are warranted to determine whether a true association exists and to elucidate the potential mechanisms underlying semaglutide-associated ocular inflammation.
Introduction
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), including semaglutide, have become a cornerstone in the management of type 2 diabetes and obesity [1]. However, their increasing use has prompted concerns regarding their ophthalmic safety, particularly given the documented increased risk of non-arteritis anterior ischaemic optic neuropathy (NAION) [2]. This study presents a unique case of inflammatory optic neuritis and scleritis in a 43-year-old female one month following commencement of semaglutide.
Case report
A 43-year-old female with a history of type 1 diabetes, obesity, and depression was commenced on 0.25 mg semaglutide weekly for weight reduction. One week following her third dose, she developed left eye retrobulbar ache and pain on eye movements. Five days later, she noted reduced vision in that eye, which worsened over the following days, prompting an urgent referral from her optometrist for ophthalmological assessment.
Her type 1 diabetes is managed with an insulin pump (haemoglobin-A1c of 6.3%) and depression well-controlled on fluoxetine. The patient has no known history of connective tissue or autoimmune disease, excluding afotrementioned type 1 diabetes. Ocular history includes right eye pars plana vitrectomy for proliferative diabetic retinopathy with tractional retinal detachment with subsequent cataract exchange and intraocular lens implantation, as well as bilateral pan retinal photocoagulation (PRP).
On examination, visual acuity in the right eye was 6/9 and left eye 6/60 with a left afferent pupillary defect. Intraocular pressure was normal, extraocular eye movements were full bilaterally with moderate left eye pain with movement. Optic nerve function testing revealed reduced red and light saturation in the left eye in comparison to the right. Ishihara colour vision testing identified correct responses on 0 plates of 14 in the left eye and 4 plates of 14 in the right.
Anterior eye examination demonstrated temporal conjunctival chemosis and mild injection of the left eye. The cornea was clear, anterior chamber deep and quiet, iris round and vitreous quiet. Left eye was phakic with mild cataract. Posterior examination revealed grade 2 disc swelling, superior retinal flame and dot blot haemorrhage, with macular oedema nasally and dense PRP peripherally.
Optical coherence tomography (OCT) confirmed significant left eye macular oedema and elevated retinal nerve fibre layer (RNFL) thickness with an average of 305 μm, in comparison to right eye of 102 μm. B scan revealed left choroidal and scleral thickening, consistent with posterior scleritis, however T sign was not present. Systemic investigations did not suggest other secondary autoimmune or infectious aetiologies. Routine blood tests (full blood count, kidney and liver function, inflammatory markers) were normal, as well as erythrocyte sedimentation rate, angiotensin converting enzyme, IgA, IgG, QuantiFERON gold assay for Mycobacterium tuberculosis, syphilis serology, antinuclear antibody, antineutrophil cytoplasmic antibody, and human leukocyte antigen (HLA)-B27. Chest radiograph showed no evidence of hilar lymphadenopathy. Computed tomography brain and orbits with contrast confirmed the diagnosis of scleritis, with diffuse thickening of the left sclera with contrast enhancement when compared to the right, as demonstrated in Fig. 1.
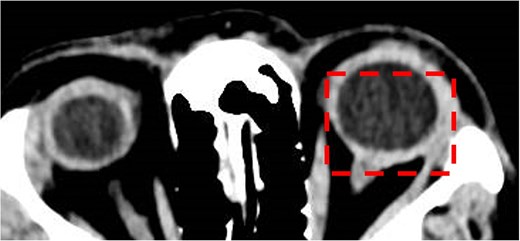
Computed tomography brain and orbits with contrast. Axial image demonstrating posterior scleritis in the left eye (dashed box).
The patient was commenced on systemic corticosteroids with 60 mg oral prednisolone once daily. Within three days, her pain improved, vision increased from 6/60 to 6/24, and OCT demonstrated reduction in RNFL thickness and macular oedema. At this time the patient disclosed having commenced semaglutide one month earlier and was advised to immediately cease the medication. After 7 days of oral prednisolone at 60 mg once daily, the dosage was weaned by 10 mg per week. Three months following presentation, the patient is taking 5 mg prednisolone, pain-free with vision returned to baseline of 6/9 and resolution of macular oedema and RNFL thickness, as demonstrated in Fig. 2.
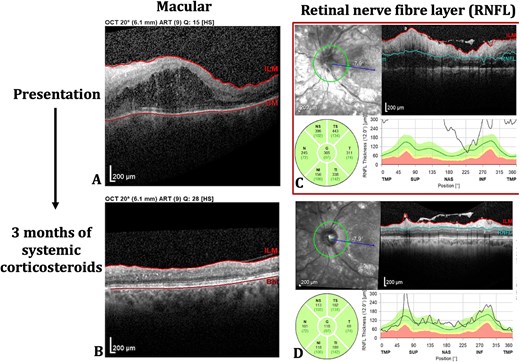
Left eye optical coherence tomography (OCT) scans of macular (A, B) and retinal nerve fibre flayer (RNFL) (C, D) at both presentation and following 3 months of systemic corticosteroids.
Discussion
GLP-1 RAs, including semaglutide, have become an integral component of weight management and type 2 diabetes treatment [1]. Systemic adverse events, such as nausea, vomiting, diarrhoea and less commonly pancreatitis [3] are documented. Ocular complications include an increased risk of NAION [2], as well as increased risk of exacerbation of retinopathy in patients with diabetic retinopathy, particularly with rapid reduction in haemoglobin-A1c levels [4]. Data on ocular adverse reactions related to GLP-1 RAs treatment remain limited, and to our knowledge, no prior cases of inflammatory optic neuritis or scleritis following commencement of GLP-1 RA use have been described. The paucity of cases in the literature may indicate that this adverse event is extremely rare.
While semaglutide is generally considered to have anti-inflammatory properties [5], isolated cases of associated inflammatory diseases, including pancreatitis [3], have been reported. In the present case, while a definitive causal link cannot be established, the onset of symptoms 4 weeks after commencing semaglutude aligns with the time to steady-state drug levels [6], raising the possibility of an association. Comprehensive investigations excluded underlying systemic aetiologies and the patient’s excellent response to corticosteroid therapy further supports the hypothesis that the condition was inflammatory in nature. Clinicians should remain vigilant in considering this as a diagnosis in patients using GLP-1 RAs, and in the absence of a known mechanism for this association, the increased risk of a rare but potentially blinding eye condition should be balanced with the many therapeutic benefits of semaglutide. Multicentre population-based cohort studies are required to investigate further.
Acknowledgements
The authors would like to acknowledge orthoptist C.G. for assisting in taking ancillary tests of patient. Patient consent was obtained prior to publication, including the publication of clinical images. Written informed consent was obtained from the patient for publication of this case report and accompanying images
Author contributions
B.G. conceived the idea for the case study. C.M. wrote the article. B.G. and A.D. revised it critically for important intellectual content. All authors read and approved the final manuscript. The manuscript has been read and approved by all the authors.
Conflict of interest statement
The authors have no proprietary or commercial interest in any materials discussed in this article. No conflicts of interest for C.M., B.G., and A.D.
Funding
No grants or funds were received for this study.
References
- renal artery stenosis
- renin-angiotensin-aldosterone system
- rotational atherectomy
- adrenal corticosteroids
- glucocorticoids
- optic neuritis
- scleritis
- eye
- mineralocorticoids
- reticular activating system
- glucagon-like peptide 1
- glucagon-like peptide-1 agonists
- nonarteritic anterior ischemic optic neuropathy
- recurrent aphthous ulcer
- semaglutide



