-
PDF
- Split View
-
Views
-
Cite
Cite
Tengfei Qi, Haiyong Ma, Xiaojiang Dai, Longying Wang, Jipei He, Xiaochen Liu, Liangping Wu, Hongbin Zhang, Reversal of Roux-en-Y anatomy via gastrogastric anastomosis and sleeve gastrectomy for refractory early dumping syndrome: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 10, October 2025, rjaf790, https://doi.org/10.1093/jscr/rjaf790
Close - Share Icon Share
Abstract
Early dumping syndrome (DS) is a common complication after Roux-en-Y gastric bypass (RYGB), with refractory cases posing significant therapeutic challenges. A 38-year-old male developed severe refractory early DS 2 years post-RYGB, confirmed by modified oral glucose tolerance test meeting Scarpellini 2020 criteria. Symptoms persisted despite maximal dietary and pharmacotherapy (acarbose, somatostatin analogues). The patient underwent laparoscopic gastrogastric anastomosis combined with sleeve gastrectomy —the first reported application for this indication. The Sigstad score decreased from 16 to 6 at 180-day follow-up, indicating complete symptom resolution. Endoscopy and imaging confirmed patent anastomosis without stenosis/leakage, and no complications occurred. This novel combined procedure is a safe and effective solution for refractory early DS post-RYGB, restoring physiological gastric emptying. Long-term outcomes require validation in larger studies.
Introduction
Roux-en-Y gastric bypass (RYGB) remains the gold standard bariatric procedure but carries a significant risk of dumping syndrome (DS) [1, 2]. Early DS specifically results from rapid nutrient delivery to the small bowel, triggering both vasomotor and gastrointestinal symptoms [3, 4]. While conservative management (e.g. dietary modifications, acarbose) suffices for most patients [5], no standardized surgical solution exists for refractory cases [6]. Current surgical interventions, such as transoral outlet reduction (TORe), demonstrate limited durability and variable efficacy; crucially, they often fail to address the underlying pathophysiology of rapid gastric emptying [6, 7]. To address this gap, we report the first application of laparoscopic gastrogastric anastomosis (GGA) combined with sleeve gastrectomy (SG) for refractory early DS post-RYGB. This novel approach aims to restore a near-physiological gastric emptying pathway via the remnant stomach and pylorus.
Case presentation
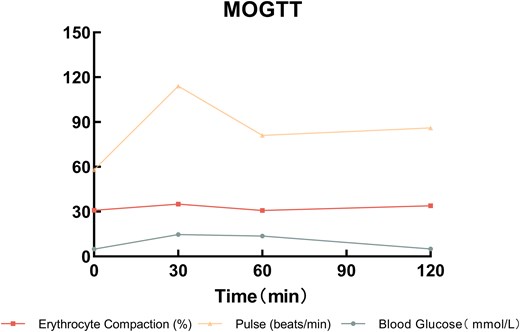
Patient history: A 38-year-old male underwent laparoscopic RYGB (Supplementary Fig. S1) for metabolic syndrome 6 years prior. Two years postoperatively, he developed refractory early DS, characterized by postprandial syncope, palpitations, and diarrhea. Diagnosis was confirmed by modified oral glucose tolerance test (MOGTT; 75 g glucose) per Scarpellini 2020 criteria [8, 9]. Detailed results are presented in Supplementary Table S1 and Fig. 1:
Hematocrit rise >3% (↑4.2%) and pulse increase >10 bpm (↑15 bpm) within 30 minutes.
Late hypoglycemia (<2.8 mmol/l; nadir 2.6 mmol/l at 180 min).
Sigstad score: 16 (Supplementary Table S2).
Maximal conservative therapy (dietary/pharmacologic) failed.
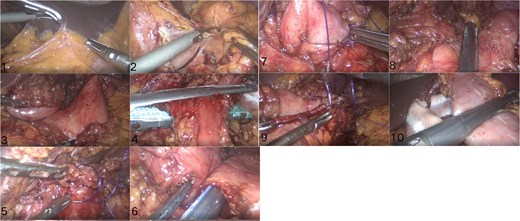
Surgical technique (Figs 2 and 3):
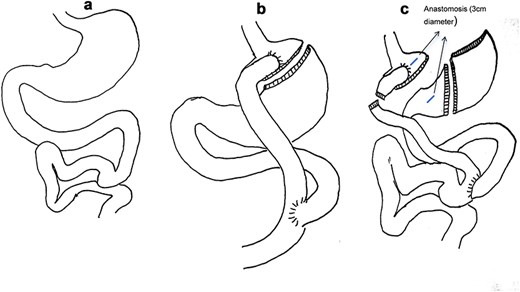
(a) Normal gastrointestinal tract structure. (b) RYGB schematic. (c) Small gastric bursa with open gastric anastomosis and sleeve gastrectomy schematic.
Key surgical steps: (1) Lysis of adhesions; (2) Division of the Roux limb 2 cm distal to the gastrojejunostomy; (3) Creation of a 3 cm gastrogastric anastomosis between the small gastric pouch and remnant stomach; (4) SG of the remnant stomach (preserving 4 cm antrum); (5) Preservation of 1 m of the jejunal Roux limb as a biliopancreatic channel to maintain enterohepatic circulation and nutrient absorption.
Rationale: Restores food transit via the pylorus, reduces gastric capacity via SG, and mitigates rapid jejunal nutrient delivery.
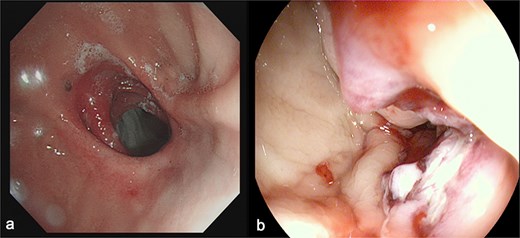
(a) Depicts the preoperative gastroscopy and (b) shows the postoperative gastroscopy.
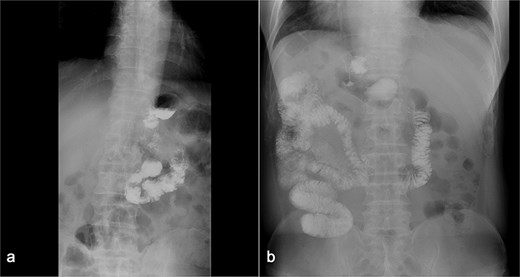
(a) Displays preoperative Gl contrast, while (b) depicts postoperative Gl contrast.
Symptoms: Sigstad score decreased from 16 (pre-op) to 6 (180 days). No dumping episodes reported.
Endoscopy/imaging: Patent GGA without stenosis/leakage (Fig. 4). Normal antral peristalsis and pyloric function (Fig. 5).
Discussion
Key findings: This study reports the first successful application of laparoscopic gastrogastric anastomosis with sleeve gastrectomy (GGA-SG) for refractory early DS post-RYGB. The procedure achieved complete symptom resolution (Sigstad score 16 → 6) at 180-day follow-up.
Mechanistic rationale:
Restoration of Pyloric Function: GGA diverts food through the remnant stomach and pylorus, restoring physiological gastric emptying and reducing rapid jejunal nutrient delivery [10, 11].
SG: Reduces gastric capacity and fundic ghrelin production, potentially mitigating hyperphagia and dumping triggers [12].
Preserved jejunal segment: The 1 m Roux limb acts as a biliopancreatic channel, minimizing malabsorption risks while maintaining GLP-1-mediated glucose homeostasis [13].
Comparison to existing surgery: TORe (endoscopic narrowing of the gastrojejunostomy) has shown mixed results [14, 15], whereas GGA + SG offers anatomical reversal of RYGB’s dumping mechanism.
Limitations: Single case, 180-day follow-up, lack of gastric emptying scintigraphy.
Future directions: Prospective cohorts assessing long-term outcomes, quality-of-life metrics (Dumping Severity Score), and standardized SG volumes/small bowel lengths.
Conclusions
Laparoscopic GGA with SG is a novel, anatomically driven solution for refractory early DS post-RYGB. This case demonstrates feasibility and short-term efficacy, warranting larger studies to validate its role in clinical practice.
Acknowledgements
The authors would like to thank all of the patients who completed the study protocol.
Conflict of interest statement
The authors have no relevant financial or no inferential interests to disclose.
Funding
This study was supported by the Guangzhou Science and Technology Plan Project (No. 2024A03J0692) and the Guangzhou Key Laboratory Construction Project (No. 2025A03J3275).
Data availability
The datasets generated or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This study was approved by the institution ethics committee. The patient signed a free and informed consent form that was previously approved and has provided informed consent for publication of the case (Process number B2020-005-02).
References
Author notes
Tengfei Qi and Haiyong Ma contributed equally to this work and should be considered co-first authors



